Label: PROTAZIL- diclazuril pellet
- NDC Code(s): 0061-4144-04
- Packager: Merck Sharp & Dohme Corp.
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- CAUTION
-
DESCRIPTION
Diclazuril, (±)-2,6-dichloro-α-(4-chlorophenyl)-4-(4,5-dihydro-3,5-dioxo-1,2,4-triazin-2(3H)-yl)benzeneacetonitrile, has a molecular formula of C17H9CI3N4O2, a molecular weight of 407.64, and a molecular structure as follows:
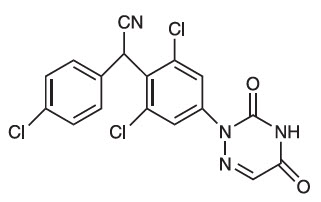
Diclazuril is an anticoccidial (antiprotozoal) compound with activity against several genera of the phylum Apicomplexa. PROTAZIL® (diclazuril) is supplied as oral pellets containing 1.56% diclazuril to be mixed as a top-dress in feed. Inert ingredients include dehydrated alfalfa meal, wheat middlings, cane molasses and propionic acid (preservative).
- INDICATIONS
-
DOSAGE AND ADMINISTRATION
Dosage: PROTAZIL® (1.56% diclazuril) is administered as a top dress in the horse's daily grain ration at a rate of 1 mg diclazuril per kg (0.45 mg diclazuril/lb) of body weight for 28 days. The quantity of PROTAZIL® necessary to deliver this dose is 64 mg pellets per kg (29 mg pellets/lb) of body weight.
Administration: To achieve this dose, weigh the horse (or use a weigh tape). Scoop up PROTAZIL® to the level (cup mark) corresponding to the dose for the horse's body weight using the following chart:
Weight Range of Horse (lb) mLs of Pellets Weight Range of Horse (lb) mLs of Pellets 275 - 524 20 1275 - 1524 60 525 - 774 30 1525 - 1774 70 775 - 1024 40 1775 - 2074 80 1025 - 1274 50 - - One 2.4-lb bucket of PROTAZIL® will treat one 1274-lb horse for 28 days. One 10-lb bucket of PROTAZIL® will treat five 1100-lb horses for 28 days.
- CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ADVERSE REACTIONS
-
CLINICAL PHARMACOLOGY
The effectiveness of diclazuril in inhibiting merozoite production of Sarcocystis neurona and S. falcatula in bovine turbinate cell cultures was studied by Lindsay and Dubey (2000).1 Diclazuril inhibited merozoite production by more than 80% in cultures of S. neurona or S. falcatula treated with 0.1 ng/mL diclazuril and greater than 95% inhibition of merozoite production (IC95) was observed when infected cultures were treated with 1.0 ng/mL diclazuril. The clinical relevance of the in vitro cell culture data has not been determined.
-
PHARMACOKINETICS IN THE HORSE
The oral bioavailability of diclazuril from the PROTAZIL® (1.56% diclazuril) Antiprotozoal Pellets at a 5 mg/kg dose rate is approximately 5%. Related diclazuril concentrations in the cerebrospinal fluid (CSF) range between 1% and 5% of the concentrations observed in the plasma. Nevertheless, based upon equine pilot study data, CSF concentrations are expected to substantially exceed the in vitro IC95 estimates for merozoite production (Dirikolu et al., 1999)2. Due to its long terminal elimination half-life in horses (approximately 43-65 hours), diclazuril accumulation occurs with once-daily dosing. Corresponding steady state blood levels are achieved by approximately Day 10 of administration.
-
EFFECTIVENESS
Two hundred and fourteen mares, stallions, and geldings of various breeds, ranging in age from 9.6 months to 30 years, were enrolled in a multi-center field study. All horses were confirmed EPM-positive based on the results of clinical examinations and laboratory testing, including CSF Western Blot analyses. Horses were administered PROTAZIL® (1.56% diclazuril) Antiprotozoal Pellets at doses of 1, 5, or 10 mg diclazuril/kg body weight as a top-dress on their daily grain ration for 28 days. The horses were then evaluated for clinical changes via a modified Mayhew neurological scale on Day 48 as follows:
- 0.
- Normal, neurological deficits not detected.
- 1.
- Neurological deficits may be detectable at normal gaits; signs exacerbated with manipulative procedures (e.g., backing, turning in tight circles, walking with head elevation, truncal swaying, etc.).
- 2.
- Neurological deficit obvious at normal gaits or posture; signs exacerbated with manipulative procedures.
- 3.
- Neurological deficit very prominent at normal gaits: horses give the impression they may fall (but do not) and buckle or fall with manipulative procedures.
- 4.
- Neurological deficit is profound at normal gait: horse frequently stumbles or trips and may fall at normal gaits or when manipulative procedures were utilized.
- 5.
- Horse is recumbent, unable to rise.
Each horse's response to treatment was compared to its pre-treatment values. Successful response to treatment was defined as clinical improvement of at least one grade by Day 48 ± conversion of CSF to Western Blot-negative status for S. neurona or achievement of Western Blot-negative CSF status without improvement of 1 ataxia grade.
Forty-two horses were initially evaluated for effectiveness and 214 horses were evaluated for safety. Clinical condition was evaluated by the clinical investigator's subjective scoring and then corroborated by evaluation of the neurological examination videotapes by a masked panel of three equine veterinarians. Although 42 horses were evaluated for clinical effectiveness, corroboration of clinical effectiveness via videotape evaluation was not possible for one horse due to missing neurologic examination videotapes. Therefore, this horse was not included in the success rate calculation. Based on the numbers of horses that seroconverted to negative Western Blot status, and the numbers of horses classified as successes by the clinical investigators, 28 of 42 horses (67%) at 1 mg/kg were considered successes. With regard to independent expert masked videotape assessments, 10 of 24 horses (42%) at 1 mg/kg were considered successes. There was no clinical difference in effectiveness among the 1, 5, and 10 mg/kg treatment group results. Adverse events were reported for two of the 214 horses evaluated for safety. In the first case, a horse was enrolled showing severe neurologic signs. Within 24 hours of dosing, the horse was recumbent, biting, and exhibiting signs of dementia. The horse died, and no cause of death was determined. In the second case, the horse began walking stiffly approximately 13 days after the start of dosing. The referring veterinarian reported that the horse had been fed grass clippings and possibly had laminitis.
-
ANIMAL SAFETY
PROTAZIL® (1.56% diclazuril) Antiprotozoal Pellets were administered to 30 horses (15 males and 15 females, ranging from 5 to 9 months of age) in a target animal safety study. Five groups of 6 horses each (3 males and 3 females) received 0, 5 (5X), 15 (15X), 25 (25X) or 50 (50X) mg diclazuril/kg (2.27mg/lb) body weight/day for 42 consecutive days as a top-dress on the grain ration of the horse. The variables measured during the study included: clinical and physical observations, body weights, food and water consumption, hematology, serum chemistry, urinalysis, fecal analysis, necropsy, organ weights, gross and histopathologic examinations. The safety of diclazuril top-dress administered to horses at 1 mg/kg once daily cannot be determined based solely on this study because of the lack of an adequate control group (control horses tested positive for the test drug in plasma and CSF). However, possible findings associated with the drug were limited to elevations in BUN, creatinine, and SDH and less than anticipated weight gain. Definitive test article-related effects were decreased grain/top-dress consumption in horses in the 50 mg/kg group.
In a second target animal safety study, PROTAZIL® (1.56% diclazuril) Antiprotozoal Pellets were administered to 24 horses (12 males and 12 females, ranging from 2 to 8 years of age). Three groups of 4 horses/sex/group received 0, 1, or 5 mg diclazuril/kg body weight/day for 42 days as a topdress on the grain ration of the horse. The variables measured during the study included physical examinations, body weights, food and water consumption, hematology, and serum chemistry. There were no test article-related findings seen during the study.
- STORAGE INFORMATION
- HOW SUPPLIED
-
REFERENCES
- Lindsay, D. S., and Dubey, J. P. 2000. Determination of the activity of diclazuril against Sarcocystis neurona and Sarcocystis falcatula in cell cultures. J. Parasitology, 86(1):164–166.
- Dirikolu, L., Lehner, F., Nattrass, C., Bentz, B. G., Woods, W. E., Carter, W. E., Karpiesiuk, W. G., Jacobs, J., Boyles, J., Harkins, J. D., Granstrom, D. E. and Tobin, T. 1999. Diclazuril in the horse: Its identification and detection and preliminary pharmacokinetics.J. Vet. Pharmacol. Therap. 22:374–379.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 1.1 kg Pellet Container Label
-
INGREDIENTS AND APPEARANCE
PROTAZIL
diclazuril pelletProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:0061-4144 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Diclazuril (UNII: K110K1B1VE) (Diclazuril - UNII:K110K1B1VE) Diclazuril 16 g in 1 kg Inactive Ingredients Ingredient Name Strength Calcium Carbonate (UNII: H0G9379FGK) Wheat Middlings (UNII: 12H8QE0WQI) Soybean (UNII: L7HT8F1ZOD) Light Mineral Oil (UNII: N6K5787QVP) Propionic Acid (UNII: JHU490RVYR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0061-4144-04 1.1 kg in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141268 11/14/2011 Labeler - Merck Sharp & Dohme Corp. (001317601) Establishment Name Address ID/FEI Business Operations Precision Science, Inc. 808156215 MANUFACTURE Establishment Name Address ID/FEI Business Operations Janssen Pharmaceutica NV 400345889 API MANUFACTURE


