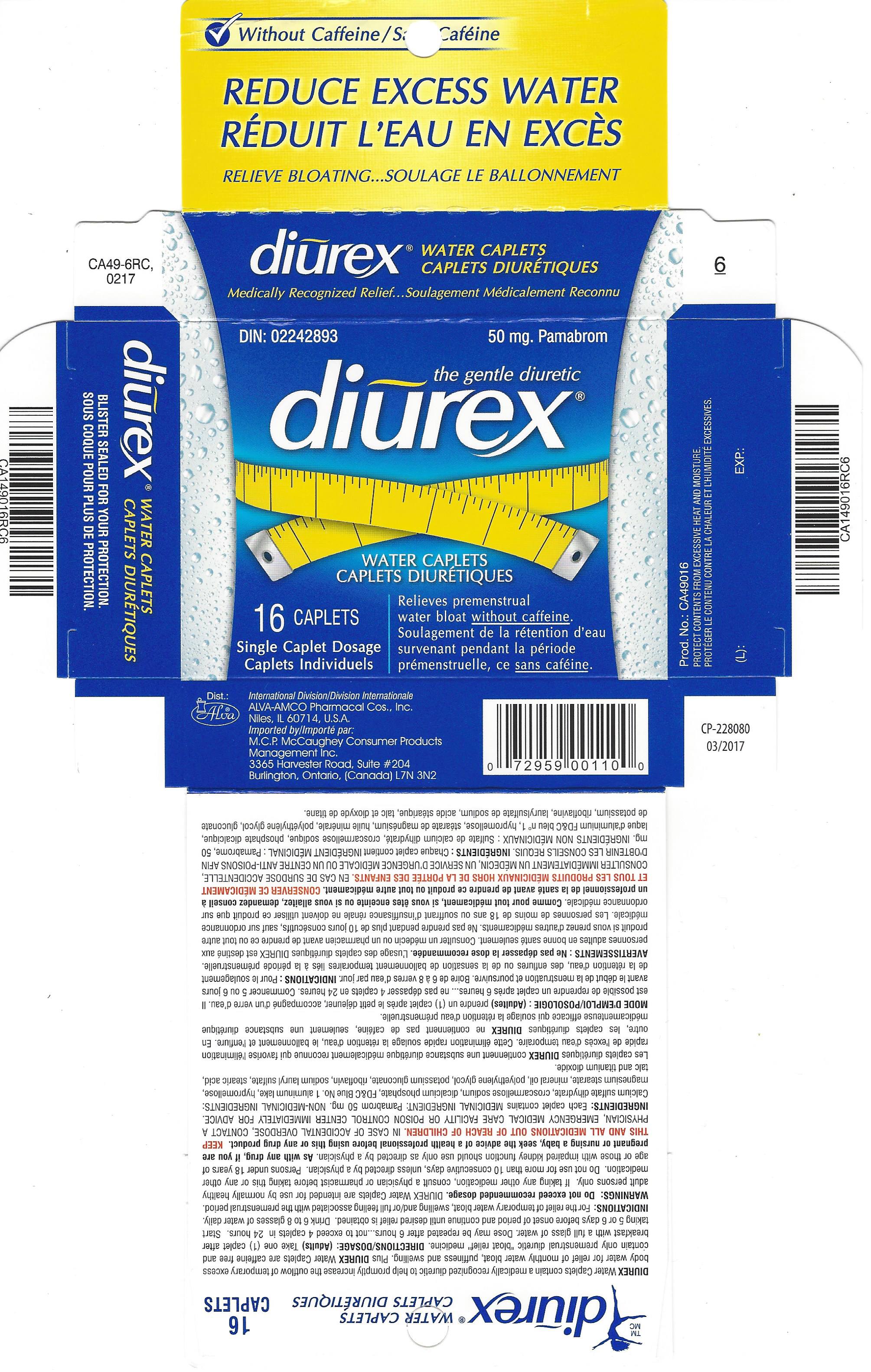
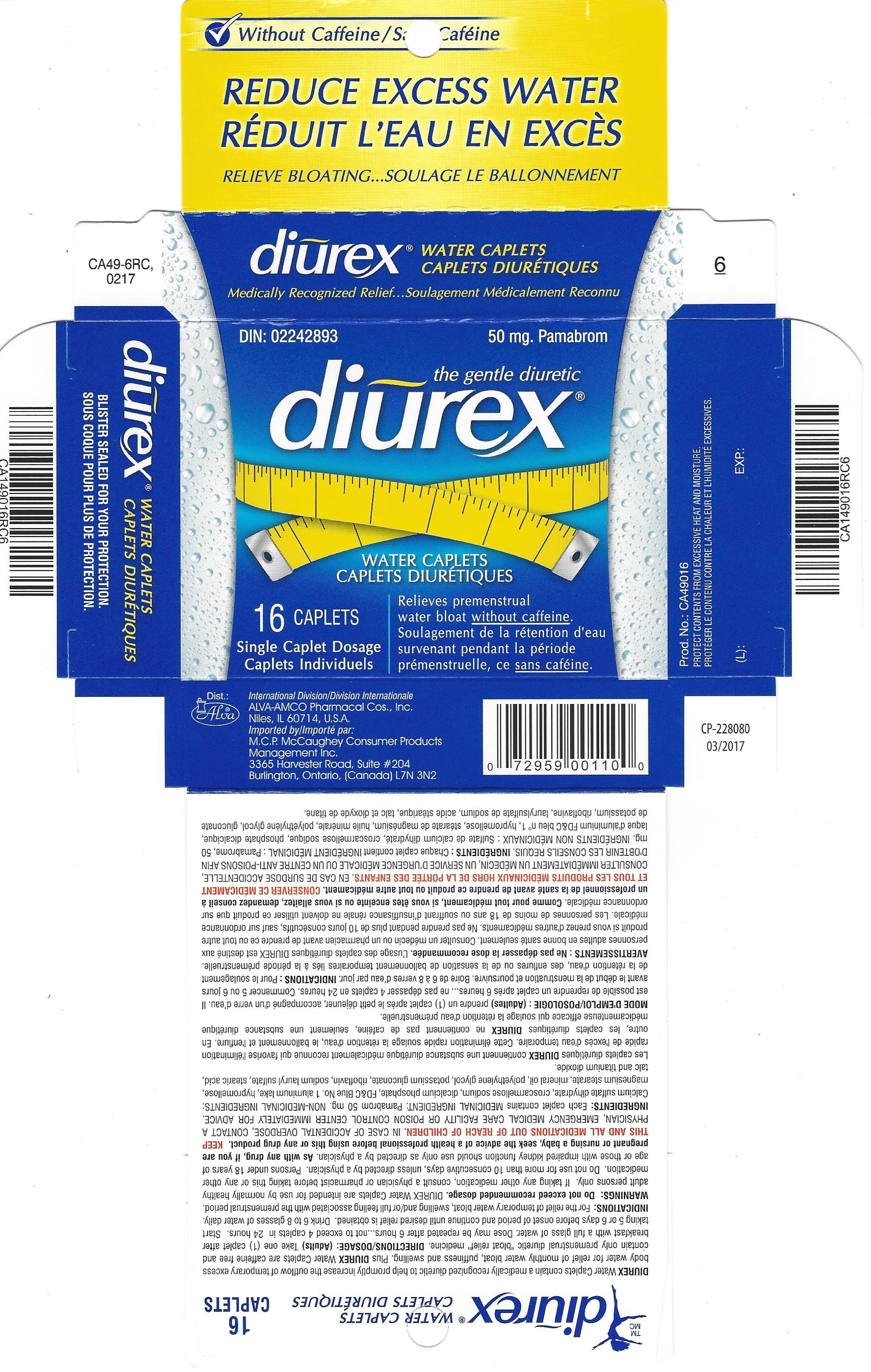
Label: DIUREX MAX- pamabrom tablet, film coated
- NDC Code(s): 52389-349-16
- Packager: Alva-Amco Pharmacal Companies, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 1, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIUREX MAX
pamabrom tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52389-349 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PAMABROM (UNII: UA8U0KJM72) (BROMOTHEOPHYLLINE - UNII:FZG87K1MQ6) PAMABROM 50 mg Inactive Ingredients Ingredient Name Strength CALCIUM SULFATE DIHYDRATE (UNII: 4846Q921YM) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) LIGHT MINERAL OIL (UNII: N6K5787QVP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POTASSIUM GLUCONATE (UNII: 12H3K5QKN9) RIBOFLAVIN (UNII: TLM2976OFR) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color blue Score no score Shape OVAL Size 15mm Flavor Imprint Code ALVA;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52389-349-16 2 in 1 CARTON 03/05/2001 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 03/05/2001 Labeler - Alva-Amco Pharmacal Companies, Inc. (042074856)