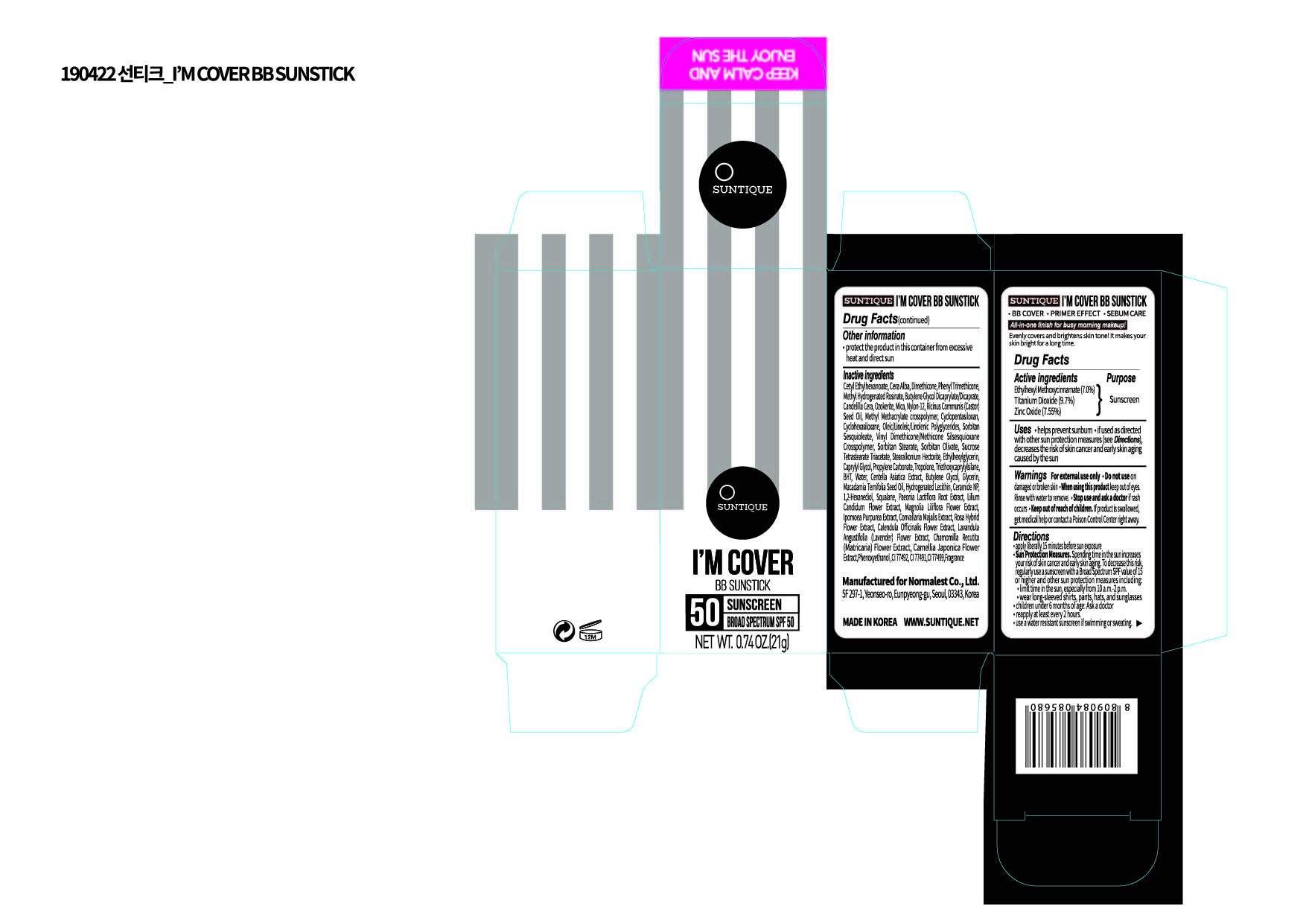
Label: IM COVER BB SUNSTICK- titanium dioxide, zinc oxide, ethylhexyl methoxycinnamate stick
-
Contains inactivated NDC Code(s)
NDC Code(s): 72284-0003-1 - Packager: Normalest Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 22, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
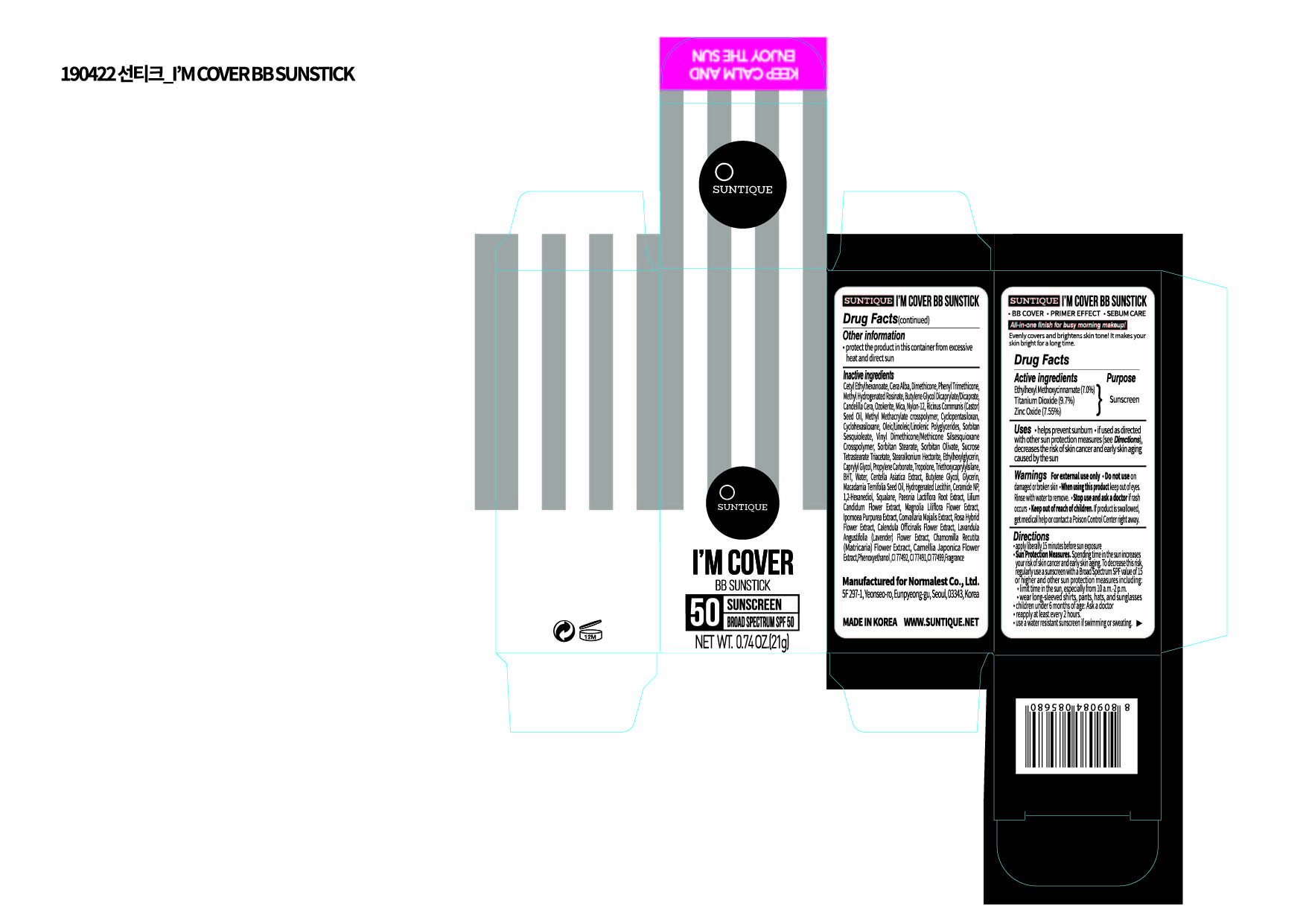 Active Ingredients
Active Ingredients
Titanium Dioxide, Zinc Oxide, Ethylhexyl Methoxycinnamate
Inactive Ingredients
Cetyl Ethylhexanoate, Cera Alba, Dimethicone, Phenyl Trimethicone, Methyl Hydrogenated Rosinate , Butylene Glycol, Dicaprylate/Dicaprate, Candelilla Cera, Ozokerite, Mica, Nylon-12, Ricinus Communis (Castor) Seed Oil, Methyl Methacrylate crosspolymer, Cyclopentasiloxan, Cyclohexasiloxane, Oleic/Linoleic/Linolenic Polyglycerides, Sorbitan Sesquioleate, Vinyl Dimethicone/Methicone Silsesquioxane Crosspolymer, Sorbitan Stearate, Sorbitan Olivate, Sucrose Tetrastearate Triacetate , Stearalkonium Hectorite, Ethylhexylglycerin, Caprylyl Glycol, Propylene Carbonate, Tropolone, Triethoxycaprylylsilane, BHT , AQUA, Centella Asiatica Extract, Butylene Glycol, Glycerin, Macadamia Ternifolia Seed Oil, Hydrogenated Lecithin, Ceramide NP, 1,2-Hexanediol, Squalane, Paeonia Lactiflora Root Extract, Lilium Candidum Flower Extract, Magnolia Liliflora Flower Extract, Ipomoea Purpurea Extract, Convallaria Majalis Extract, Rosa Hybrid Flower Extract, Calendula Officinalis Flower Extract, Lavandula Angustifolia Flower Extract, Chamomilla Recutita Flower Extract , Camellia Japonica Flower Extract, Phenoxyethanol , CI 77492, CI 77491, CI 77499, Fragrance
-
INGREDIENTS AND APPEARANCE
IM COVER BB SUNSTICK
titanium dioxide, zinc oxide, ethylhexyl methoxycinnamate stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72284-0003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 2.037 g in 21 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1.585 g in 21 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.47 g in 21 g Inactive Ingredients Ingredient Name Strength CONVALLARIA MAJALIS (UNII: QHH4HVF5QE) CAMELLIA JAPONICA FLOWER (UNII: KUB8101TNF) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) NYLON-12 (UNII: 446U8J075B) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) SUCROSE TETRASTEARATE TRIACETATE (UNII: 1K7LBQ045N) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) PROPYLENE CARBONATE (UNII: 8D08K3S51E) TROPOLONE (UNII: 7L6DL16P1T) CETYL ETHYLHEXANOATE (UNII: 134647WMX4) DIMETHICONE (UNII: 92RU3N3Y1O) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) METHYL HYDROGENATED ROSINATE (UNII: 13DHA19W9N) CENTELLA ASIATICA (UNII: 7M867G6T1U) SQUALANE (UNII: GW89575KF9) IPOMOEA PURPUREA TOP (UNII: XMQ0V9812O) LAVANDULA ANGUSTIFOLIA FLOWER (UNII: 19AH1RAF4M) PHENOXYETHANOL (UNII: HIE492ZZ3T) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) SORBITAN OLIVATE (UNII: MDL271E3GR) CAPRYLYL GLYCOL (UNII: 00YIU5438U) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) MICA (UNII: V8A1AW0880) RICINUS COMMUNIS SEED (UNII: 7EK4SFN1TX) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: 657L0NC5MT) WHITE WAX (UNII: 7G1J5DA97F) BUTYLENE GLYCOL DICAPRYLATE/DICAPRATE (UNII: 75D21FL1PI) CANDELILLA WAX (UNII: WL0328HX19) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) GLYCERIN (UNII: PDC6A3C0OX) MACADAMIA OIL (UNII: 515610SU8C) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) PAEONIA LACTIFLORA ROOT (UNII: 3Z3866YW6P) LILIUM CANDIDUM FLOWER (UNII: COV655U2CJ) MAGNOLIA LILIIFLORA FLOWER (UNII: SVM28292LH) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CHAMOMILE (UNII: FGL3685T2X) FERRIC OXIDE RED (UNII: 1K09F3G675) FERROSOFERRIC OXIDE (UNII: XM0M87F357) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CERAMIDE NP (UNII: 4370DF050B) Product Characteristics Color Score Shape ROUND Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72284-0003-1 1 in 1 BOX 04/24/2019 1 21 g in 1 CONTAINER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 04/24/2019 Labeler - Normalest Co., Ltd. (694812877) Registrant - Normalest Co., Ltd. (694812877) Establishment Name Address ID/FEI Business Operations Normalest Co., Ltd. 694812877 manufacture(72284-0003)