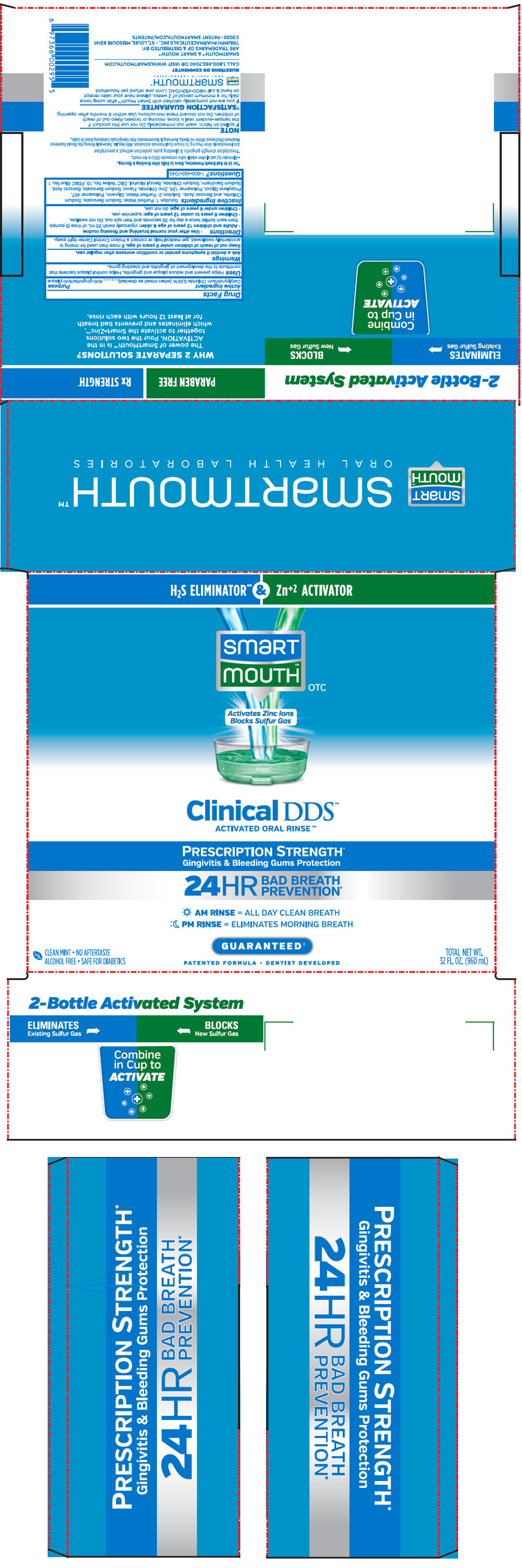
Label: SMARTMOUTH MOUTHWASH CLINICAL DDS FORMULA- cetylpyridinium chloride kit
- NDC Code(s): 76357-285-01
- Packager: Triumph Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
-
Inactive Ingredients
Solution 1: Purified Water, Sodium Benzoate, Sodium Chlorite, and Benzoic Acid
Solution 2: Purified Water, Glycerin, Poloxamer 407, Propylene Glycol, Poloxamer 124, Zinc Chloride, Flavor, Sodium Benzoate, Benzoic Acid, Sodium Saccharin, Sodium Chloride, Benzyl Alcohol, D&C Yellow No. 10, FD&C Blue No. 1
- Questions or comments
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - Kit Cello Pack
H2S ELIMINATOR∞ & Zn+2 ACTIVATOR
smart
mouth™
OTCActivates Zinc Ions
Blocks Sulfur GasClinical DDS™
ACTIVATED ORAL RINSE™PRESCRIPTION STRENGTH+
Gingivitis & Bleeding Gums Protection24HR
BAD BREATH
PREVENTION*AM RINSE = ALL DAY CLEAN BREATH
PM RINSE = ELIMINATES MORNING BREATHGUARANTEED†
PATENTED FORMULA • DENTIST DEVELOPED
CLEAN MINT • NO AFTERTASTE
ALCOHOL FREE • SAFE FOR DIABETICSTOTAL NET WT.
32 FL. OZ. (960 mL)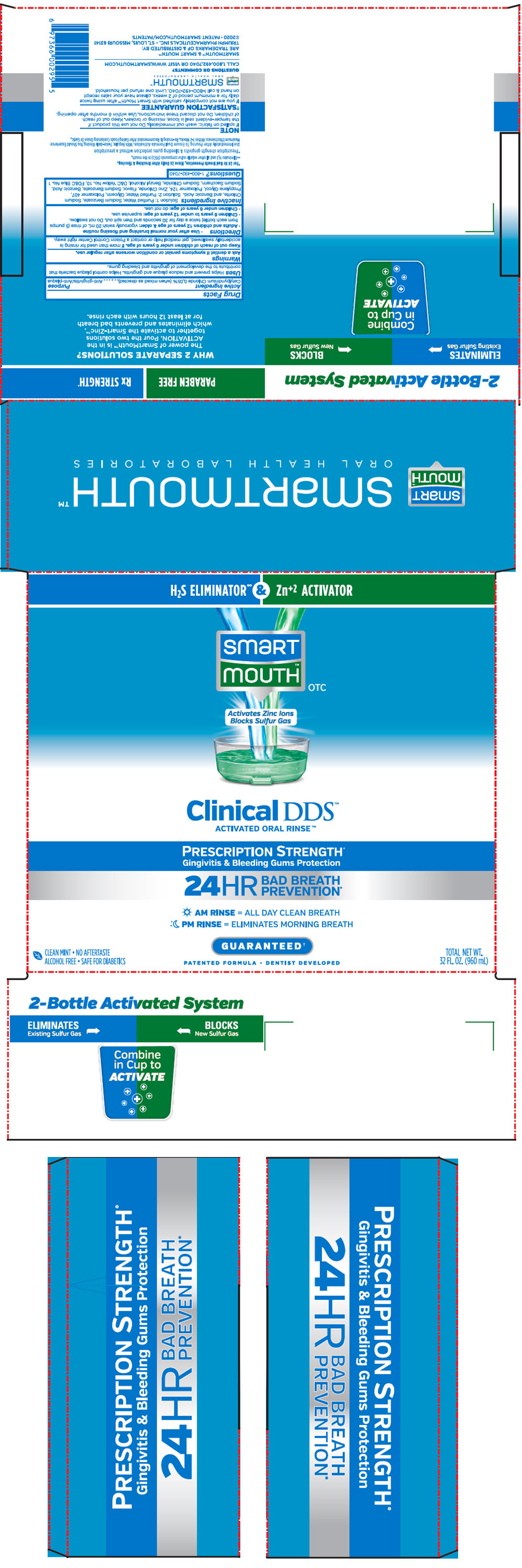
-
INGREDIENTS AND APPEARANCE
SMARTMOUTH MOUTHWASH CLINICAL DDS FORMULA
cetylpyridinium chloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76357-285 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76357-285-01 1 in 1 CELLO PACK; Type 0: Not a Combination Product 10/01/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 480 mL Part 2 1 BOTTLE, PLASTIC 480 mL Part 1 of 2 SMARTMOUTH CLINICAL DDS SOLUTION 1
mouthwashes and breath fresheners (liquids and sprays)Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR Sodium Benzoate (UNII: OJ245FE5EU) INGR Sodium Chlorite (UNII: G538EBV4VF) INGR Benzoic Acid (UNII: 8SKN0B0MIM) Product Characteristics color WHITE C48325 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 480 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 10/01/2018 Part 2 of 2 SMARTMOUTH CLINICAL DDS SOLUTION 2 MINT
cetylpyridinium chloride liquidProduct Information Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Cetylpyridinium Chloride (UNII: D9OM4SK49P) (Cetylpyridinium - UNII:CUB7JI0JV3) Cetylpyridinium Chloride 500 mg in 20 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Poloxamer 407 (UNII: TUF2IVW3M2) Propylene Glycol (UNII: 6DC9Q167V3) Poloxamer 124 (UNII: 1S66E28KXA) Zinc Chloride (UNII: 86Q357L16B) Sodium Benzoate (UNII: OJ245FE5EU) Benzoic Acid (UNII: 8SKN0B0MIM) Saccharin Sodium (UNII: SB8ZUX40TY) Sodium Chloride (UNII: 451W47IQ8X) Benzyl Alcohol (UNII: LKG8494WBH) D&C Yellow No. 10 (UNII: 35SW5USQ3G) FD&C Blue No. 1 (UNII: H3R47K3TBD) Product Characteristics Color GREEN Score Shape Size Flavor MINT (mint vanilla) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 480 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part356 10/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part356 10/01/2018 Labeler - Triumph Pharmaceuticals Inc. (017853461)