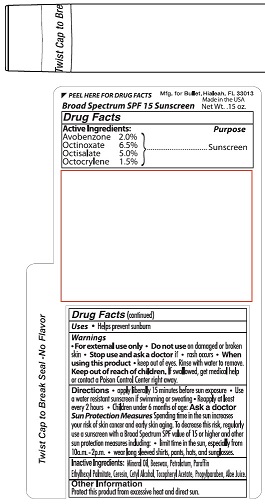
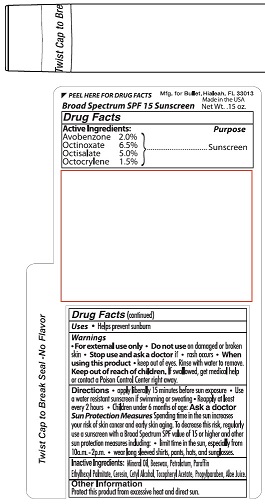
Label: BULLET LINE- lip balm spf 15 stick
-
NDC Code(s):
65692-1006-1,
65692-1007-1,
65692-1008-1,
65692-1009-1, view more65692-1010-1, 65692-1011-1, 65692-1012-1
- Packager: Raining Rose, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
-
OTHER SAFETY INFORMATION
Sun Protection Measures Spending time in the sun increases your
risk of skin cancer and early skin aging. To decrease this risk, regularly
use a sunscreen with a Broad-Spectrum SPF value of 15 or higher and
other protection measures including: • limit time in the sun, especially from
10a.m. - 2p.m. • wear long sleeved shirts, pants, hat, and sunglasses. - INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- Package Labeling
-
INGREDIENTS AND APPEARANCE
BULLET LINE
lip balm spf 15 stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65692-1006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.0 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.5 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5.0 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) WHITE WAX (UNII: 7G1J5DA97F) YELLOW WAX (UNII: 2ZA36H0S2V) PETROLATUM (UNII: 4T6H12BN9U) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CERESIN (UNII: Q1LS2UJO3A) CETYL ALCOHOL (UNII: 936JST6JCN) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65692-1006-1 4 g in 1 TUBE; Type 0: Not a Combination Product 02/18/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/18/2020 BULLET LINE
lip balm spf 15 stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65692-1007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.0 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.5 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5.0 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) YELLOW WAX (UNII: 2ZA36H0S2V) PETROLATUM (UNII: 4T6H12BN9U) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CERESIN (UNII: Q1LS2UJO3A) CETYL ALCOHOL (UNII: 936JST6JCN) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65692-1007-1 4 g in 1 TUBE; Type 0: Not a Combination Product 02/18/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/18/2020 BULLET LINE
lip balm spf 15 stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65692-1008 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.0 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.5 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5.0 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) YELLOW WAX (UNII: 2ZA36H0S2V) PETROLATUM (UNII: 4T6H12BN9U) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CERESIN (UNII: Q1LS2UJO3A) CETYL ALCOHOL (UNII: 936JST6JCN) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65692-1008-1 4 g in 1 TUBE; Type 0: Not a Combination Product 02/18/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/18/2020 BULLET LINE
lip balm spf 15 stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65692-1009 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.0 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.5 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5.0 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) YELLOW WAX (UNII: 2ZA36H0S2V) PETROLATUM (UNII: 4T6H12BN9U) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CERESIN (UNII: Q1LS2UJO3A) CETYL ALCOHOL (UNII: 936JST6JCN) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65692-1009-1 4 g in 1 TUBE; Type 0: Not a Combination Product 02/18/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/18/2020 BULLET LINE
lip balm spf 15 stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65692-1010 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.0 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.5 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5.0 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) YELLOW WAX (UNII: 2ZA36H0S2V) PETROLATUM (UNII: 4T6H12BN9U) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CERESIN (UNII: Q1LS2UJO3A) CETYL ALCOHOL (UNII: 936JST6JCN) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color Score Shape Size Flavor STRAWBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65692-1010-1 4 g in 1 TUBE; Type 0: Not a Combination Product 02/18/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/18/2020 BULLET LINE
lip balm spf 15 stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65692-1011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.0 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.5 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5.0 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) YELLOW WAX (UNII: 2ZA36H0S2V) PETROLATUM (UNII: 4T6H12BN9U) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CERESIN (UNII: Q1LS2UJO3A) CETYL ALCOHOL (UNII: 936JST6JCN) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color Score Shape Size Flavor TROPICAL FRUIT PUNCH (TROPICAL) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65692-1011-1 4 g in 1 TUBE; Type 0: Not a Combination Product 02/18/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/18/2020 BULLET LINE
lip balm spf 15 stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65692-1012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.0 mg in 1 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 6.5 mg in 1 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5.0 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) YELLOW WAX (UNII: 2ZA36H0S2V) PETROLATUM (UNII: 4T6H12BN9U) PARAFFIN (UNII: I9O0E3H2ZE) ETHYLHEXYL PALMITATE (UNII: 2865993309) CERESIN (UNII: Q1LS2UJO3A) CETYL ALCOHOL (UNII: 936JST6JCN) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) PROPYLPARABEN (UNII: Z8IX2SC1OH) ALOE VERA LEAF (UNII: ZY81Z83H0X) Product Characteristics Color Score Shape Size Flavor VANILLA Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65692-1012-1 4 g in 1 TUBE; Type 0: Not a Combination Product 02/18/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/18/2020 Labeler - Raining Rose, Inc (083819404)