Label: METZ SANI-SHIELD IODINE BARRIER TEAT DIP- iodine solution
-
NDC Code(s):
86067-0122-1,
86067-0122-2,
86067-0122-3,
86067-0122-4, view more86067-0122-5, 86067-0122-6
- Packager: Surpass Chemical Company, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
-
INSTRUCTIONS FOR USE
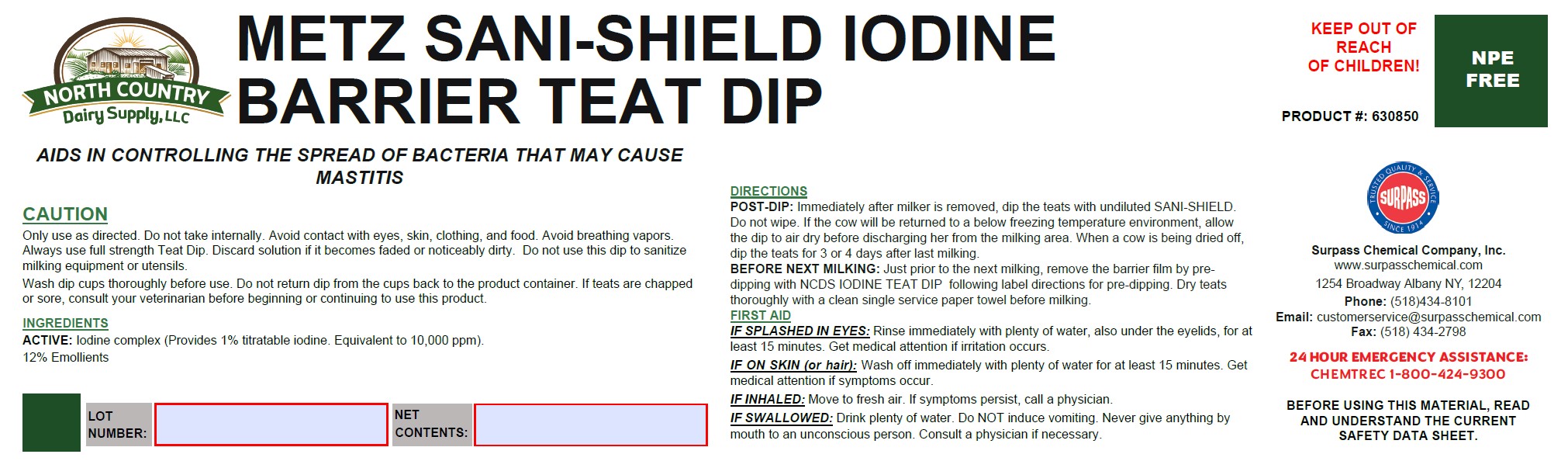
DIRECTIONS:
POST-DIP: Immediately after milker is removed, dip the teats with undiluted SANI-SHIELD. Do not wipe. If the cow will be
returned to a below freezing temperature environment, allow the dip to air dry before discharging her from the milking area.
When a cow is being dried off, dip the teats for 3 or 4 days after last milking.
BEFORE THE NEXT MILKING: Just prior to the next milking, remove the barrier film by pre-dipping with NCDS IODINE
TEAT DIP following label directions for pre-dipping. Dry teats thoroughly with a clean single service paper towel before
milking. -
GENERAL PRECAUTIONS
CAUTION:
Only use as directed. Do not take internally. Avoid contact with eyes, skin, clothing, and food. Avoid breathing vapors.
Always use full strength Teat Dip. Discard solution if it becomes faded or noticeably dirty. Do not use this dip to sanitize
milking equipment or utensils.
Wash dip cups thoroughly before use. Do not return dip from the cups back to the product container. If teats are chapped or
sore, consult your veterinarian before beginning or continuing to use this product. -
PRECAUTIONS
PRECAUTIONARY STATEMENTS
Prevention: Do not breathe dust/fume/gas/mist/vapors/spray Wash face, hands and any exposed skin thoroughly after
handling Do not eat, drink or smoke when using this product
Response: Get medical advice/attention if you feel unwell
Disposal: Dispose of contents/container to an approved waste disposal plant -
OTHER SAFETY INFORMATION
FIRST AID:
IF SPLASHED IN EYES: Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. Get
medical attention if irritation occurs.
IF ON SKIN (or hair): Wash off immediately with plenty of water for at least 15 minutes. Get medical attention if
symptoms occur.
IF INHALED: Move to fresh air. If symptoms persist, call a physician.
IF SWALLOWED: Drink plenty of water. Do NOT induce vomiting. Never give anything by mouth to an unconscious
person. Consult a physician if necessary. - ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METZ SANI-SHIELD IODINE BARRIER TEAT DIP
iodine solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86067-0122 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength iodine (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) iodine 0.04724 kg in 1 kg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 0.87076 kg in 1 kg C9-11 PARETH-8 (UNII: 80E6PSE1XL) 0.02 kg in 1 kg ALOE VERA FLOWER (UNII: 575DY8C1ER) 0.0005 kg in 1 kg GLYCERIN (UNII: PDC6A3C0OX) 0.05 kg in 1 kg SODIUM BICARBONATE (UNII: 8MDF5V39QO) 0.0025 kg in 1 kg XANTHAN GUM (UNII: TTV12P4NEE) 0.003 kg in 1 kg BUTYLENE GLYCOL (UNII: 3XUS85K0RA) 0.006 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86067-0122-2 4 in 1 BOX 1 NDC:86067-0122-1 3.87 kg in 1 JUG 2 NDC:86067-0122-3 19.05 kg in 1 PAIL 3 NDC:86067-0122-4 58.06 kg in 1 DRUM 4 NDC:86067-0122-5 116.12 kg in 1 DRUM 5 NDC:86067-0122-6 213.19 kg in 1 DRUM Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/15/2023 Labeler - Surpass Chemical Company, Inc. (002075133) Establishment Name Address ID/FEI Business Operations Surpass Chemical Company, Inc. 002075133 manufacture, api manufacture