Label: SYMTUZA- darunavir, cobicistat, emtricitabine, and tenofovir alafenamide tablet, film coated
- NDC Code(s): 59676-800-30, 59676-800-99
- Packager: Janssen Products LP
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated July 23, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SYMTUZA safely and effectively. See full prescribing information for SYMTUZA.
SYMTUZA ®(darunavir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets, for oral use
Initial U.S. Approval: 2018WARNING: POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B
See full prescribing information for complete boxed warning.
Severe acute exacerbations of hepatitis B (HBV) have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued products containing emtricitabine and/or tenofovir disoproxil fumarate (TDF), and may occur with discontinuation of SYMTUZA. Hepatic function should be monitored closely in these patients. If appropriate, anti-hepatitis B therapy may be warranted. ( 5.1)
RECENT MAJOR CHANGES
Contraindications ( 4) 04/2022 INDICATIONS AND USAGE
SYMTUZA is a four-drug combination of darunavir (DRV), a human immunodeficiency virus (HIV-1) protease inhibitor, cobicistat (COBI), a CYP3A inhibitor, and emtricitabine (FTC) and tenofovir alafenamide (TAF), both HIV-1 nucleoside analog reverse transcriptase inhibitors, and is indicated as a complete regimen for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 40 kg:
- who have no prior antiretroviral treatment history or
- who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen for at least 6 months and have no known substitutions associated with resistance to darunavir or tenofovir. ( 1)
DOSAGE AND ADMINISTRATION
Testing: Prior to or when initiating SYMTUZA, test patients for HBV infection.
Prior to or when initiating SYMTUZA, and during treatment with SYMTUZA, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. ( 2.1)
Recommended dosage: One tablet taken once daily with food in adults and pediatric patients, weighing at least 40 kg. ( 2.2)
Renal Impairment:SYMTUZA is not recommended in patients with estimated creatinine clearance below 30 mL/min. ( 2.3)
Hepatic Impairment: SYMTUZA is not recommended in patients with severe hepatic impairment. ( 2.4)
DOSAGE FORMS AND STRENGTHS
Tablets: 800 mg of darunavir, 150 mg of cobicistat, 200 mg of emtricitabine, and 10 mg of tenofovir alafenamide (equivalent to 11.2 mg of tenofovir alafenamide fumarate). ( 3)
CONTRAINDICATIONS
SYMTUZA is contraindicated to be co-administered with certain drugs for which altered plasma concentrations are associated with serious and/or life-threatening events or which may lead to loss of therapeutic effect of SYMTUZA and development of resistance. ( 4)
WARNINGS AND PRECAUTIONS
- Drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) including some fatalities can occur with SYMTUZA. Monitor liver function before and during therapy, especially in patients with underlying chronic hepatitis, cirrhosis, or in patients who have pre-treatment elevations of transaminases. ( 5.2)
- Severe skin reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms, and acute generalized exanthematous pustulosis may occur with SYMTUZA. Discontinue treatment if severe skin reaction develops. ( 5.3)
- Patients receiving SYMTUZA may develop new onset or exacerbations of immune reconstitution syndrome. ( 5.5)
- Monitor in patients with a known sulfonamide allergy. ( 5.7)
- Discontinue treatment in patients who develop symptoms or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity. ( 5.8)
- Patients receiving SYMTUZA may develop new onset or exacerbation of diabetes mellitus/hyperglycemia and redistribution/accumulation of body fat. ( 5.9, 5.10)
- Patients with hemophilia may develop increase bleeding events. ( 5.11)
ADVERSE REACTIONS
The most common adverse reactions (all grades, incidence greater than or equal to 2%) were diarrhea, rash, nausea, fatigue, headache, abdominal discomfort, and flatulence. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Products, LP at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation of SYMTUZA
2.2 Recommended Dosage
2.3 Not Recommended in Patients with Severe Renal Impairment
2.4 Not Recommended in Patients with Severe Hepatic Impairment
2.5 Not Recommended During Pregnancy
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
5. WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
5.2 Hepatotoxicity
5.3 Severe Skin Reactions
5.4 Risk of Serious Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
5.5 Immune Reconstitution Syndrome
5.6 New Onset or Worsening Renal Impairment
5.7 Sulfa Allergy
5.8 Lactic Acidosis/Severe Hepatomegaly with Steatosis
5.9 Diabetes Mellitus/Hyperglycemia
5.10 Fat Redistribution
5.11 Hemophilia
6. ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7. DRUG INTERACTIONS
7.1 Not Recommended With Other Antiretroviral Medications
7.2 Potential for SYMTUZA to Affect Other Drugs
7.3 Potential for Other Drugs to Affect SYMTUZA
7.4 Drugs Affecting Renal Function
7.5 Significant Drug Interactions
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10. OVERDOSAGE
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14. CLINICAL STUDIES
14.1 Clinical Trial Results in Subjects with HIV-1 Infection with no Prior Antiretroviral Treatment History
14.2 Clinical Trial Results in Virologically-Suppressed Subjects with HIV-1 Infection Who Switched to SYMTUZA
14.3 Clinical Trial Results in Pediatric Subjects with HIV-1 Infection
16. HOW SUPPLIED/STORAGE AND HANDLING
17. PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: POST TREATMENT ACUTE EXACERBATION OF HEPATITIS B
Severe acute exacerbations of hepatitis B (HBV) have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued products containing emtricitabine and/or tenofovir disoproxil fumarate (TDF), and may occur with discontinuation of SYMTUZA. Closely monitor hepatic function with both clinical and laboratory follow-up for at least several months in patients who are coinfected with HIV-1 and HBV and discontinue SYMTUZA. If appropriate, anti-hepatitis B therapy may be warranted [see Warnings and Precautions (5.1)] .
-
1. INDICATIONS AND USAGE
SYMTUZA is indicated as a complete regimen for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and pediatric patients weighing at least 40 kg:
- who have no prior antiretroviral treatment history or
- who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen for at least 6 months and have no known substitutions associated with resistance to darunavir or tenofovir.
-
2. DOSAGE AND ADMINISTRATION
2.1 Testing Prior to Initiation of SYMTUZA
Prior to or when initiating SYMTUZA, test patients for hepatitis B (HBV) virus infection [see Warnings and Precautions (5.1)] .
Prior to or when initiating SYMTUZA, and during treatment with SYMTUZA, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus [see Warnings and Precautions (5.6)] .
2.2 Recommended Dosage
SYMTUZA is a four-drug fixed-dose combination product containing 800 mg of darunavir (DRV), 150 mg of cobicistat (COBI), 200 mg of emtricitabine (FTC), and 10 mg of tenofovir alafenamide (TAF). The recommended dosage of SYMTUZA is one tablet taken orally once daily with food in adults and pediatric patients weighing at least 40 kg. For patients who are unable to swallow the whole tablet, SYMTUZA may be split into two pieces using a tablet-cutter, and the entire dose should be consumed immediately after splitting [see Clinical Pharmacology (12.3)] .
2.3 Not Recommended in Patients with Severe Renal Impairment
SYMTUZA is not recommended in patients with creatinine clearance below 30 mL per minute [see Use in Specific Populations (8.6)] .
2.4 Not Recommended in Patients with Severe Hepatic Impairment
SYMTUZA is not recommended for use in patients with severe hepatic impairment (Child-Pugh Class C) [see Use in Specific Populations (8.7)] .
2.5 Not Recommended During Pregnancy
SYMTUZA is not recommended during pregnancy because of substantially lower exposures of darunavir and cobicistat during the second and third trimesters [see Use in Specific Populations (8.1)and Clinical Pharmacology (12.3)].
SYMTUZA should not be initiated in pregnant individuals. An alternative regimen is recommended for those who become pregnant during therapy with SYMTUZA.
-
3. DOSAGE FORMS AND STRENGTHS
Each SYMTUZA tablet contains darunavir ethanolate equivalent to 800 mg of darunavir, 150 mg of cobicistat, 200 mg of emtricitabine (FTC), and tenofovir alafenamide fumarate equivalent to 10 mg of tenofovir alafenamide (TAF). The yellow to yellowish-brown, capsule-shaped, film-coated tablet is debossed with "8121" on one side and "JG" on the other side.
-
4. CONTRAINDICATIONS
Darunavir and cobicistat are both inhibitors of the cytochrome P450 3A (CYP3A) isoform. SYMTUZA should not be co-administered with medicinal products that are highly dependent on CYP3A for clearance and for which increased plasma concentrations are associated with serious and/or life-threatening events (narrow therapeutic index). Darunavir and cobicistat are both substrates of the cytochrome P450 3A (CYP3A) isoform. Co-administration of SYMTUZA with CYP3A inducers is expected to lower plasma concentrations of darunavir and cobicistat which may lead to loss of efficacy of darunavir and development of resistance. Examples of drugs that are contraindicated for co-administration with SYMTUZA due to the potential for serious and/or life-threatening events or loss of therapeutic effect [see Drug Interactions (7.5)] are listed below.
- Alpha 1-adrenoreceptor antagonist: alfuzosin
- Anticonvulsants: carbamazepine, phenobarbital, phenytoin
- Anti-gout: colchicine, in patients with renal and/or hepatic impairment
- Antimycobacterial: rifampin
- Antipsychotics: lurasidone, pimozide
- Cardiac Disorders: dronedarone, ivabradine, ranolazine
- Ergot derivatives, e.g., dihydroergotamine, ergotamine, methylergonovine
- Herbal product: St. John's wort ( Hypericum perforatum)
- Hepatitis C direct acting antiviral: elbasvir/grazoprevir
- Lipid modifying agents: lomitapide, lovastatin, simvastatin
- Opioid Antagonist: naloxegol
- PDE-5 inhibitor: sildenafil when used for treatment of pulmonary arterial hypertension
- Sedatives/hypnotics: orally administered midazolam, triazolam
-
5. WARNINGS AND PRECAUTIONS
5.1 Severe Acute Exacerbation of Hepatitis B in Patients Coinfected with HIV-1 and HBV
Patients with HIV-1 should be tested for the presence of chronic hepatitis B virus before initiating antiretroviral therapy [see Dosage and Administration (2.1)] . Severe acute exacerbations of hepatitis B (e.g., liver decompensation and liver failure) have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued products containing emtricitabine and/or tenofovir disoproxil fumarate, and may occur with discontinuation of SYMTUZA. Patients coinfected with HIV-1 and HBV who discontinue SYMTUZA should be closely monitored with both clinical and laboratory follow-up for at least several months after stopping treatment. If appropriate, anti-hepatitis B therapy may be warranted, especially in patients with advanced liver disease or cirrhosis, since post-treatment exacerbation of hepatitis may lead to hepatic decompensation and liver failure.
5.2 Hepatotoxicity
Drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) has been reported in clinical trials with darunavir, a component of SYMTUZA. Patients with pre-existing liver dysfunction, including chronic active hepatitis B or C, have an increased risk for liver function abnormalities including severe hepatic adverse reactions.
Post-marketing cases of liver injury, including some fatalities, have been reported with darunavir. These have generally occurred in patients with advanced HIV-1 disease taking multiple concomitant medications, having co-morbidities including hepatitis B or C co-infection, and/or developing immune reconstitution syndrome. A causal relationship with darunavir therapy has not been established.
Appropriate laboratory testing should be conducted prior to initiating therapy with SYMTUZA and patients should be monitored during treatment as clinically appropriate. Increased AST/ALT monitoring should be considered in patients with underlying chronic hepatitis, cirrhosis, or in patients who have pre-treatment elevations of transaminases, especially during the first several months of SYMTUZA treatment.
Evidence of new or worsening liver dysfunction (including clinically significant elevation of liver enzymes and/or symptoms such as fatigue, anorexia, nausea, jaundice, dark urine, liver tenderness, hepatomegaly) should prompt consideration of interruption or discontinuation of SYMTUZA.
5.3 Severe Skin Reactions
In patients receiving darunavir, a component of SYMTUZA, severe skin reactions may occur. These include conditions accompanied by fever and/or elevations of transaminases. Stevens-Johnson syndrome was reported with darunavir co-administered with cobicistat in clinical trials at a rate of 0.1%. During darunavir post-marketing experience, toxic epidermal necrolysis, drug rash with eosinophilia and systemic symptoms (DRESS), and acute generalized exanthematous pustulosis have been reported. Discontinue SYMTUZA immediately if signs or symptoms of severe skin reactions develop. These can include but are not limited to severe rash or rash accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, hepatitis, and/or eosinophilia.
Rash events of any cause and any grade occurred in 15% of subjects with no prior antiretroviral treatment history treated with SYMTUZA in the AMBER trial [see Adverse Reactions (6.1)] . Rash events were mild-to-moderate, often occurring within the first four weeks of treatment and resolving with continued dosing. The discontinuation rate due to rash in subjects using SYMTUZA was 2%.
5.4 Risk of Serious Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of SYMTUZA and other drugs may result in known or potentially significant drug interactions which may lead to [see Contraindications (4)and Drug Interactions (7.5)] :
- Clinically significant adverse reactions from greater exposures of concomitant drugs.
- Clinically significant adverse reactions from greater exposures of SYMTUZA.
- Loss of therapeutic effect of the concomitant drugs from lower exposures of active metabolite(s).
- Loss of therapeutic effect of SYMTUZA and possible development of resistance from lower exposures of SYMTUZA.
See Table 4for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during SYMTUZA therapy; review concomitant medications during SYMTUZA therapy; and monitor for the adverse reactions associated with concomitant medications [see Contraindications (4)and Drug Interactions (7)] .
When used with concomitant medications, SYMTUZA, which contains darunavir boosted with cobicistat, may result in different drug interactions than those observed or expected with darunavir co-administered with ritonavir. Complex or unknown mechanisms of drug interactions preclude extrapolation of drug interactions with darunavir co-administered with ritonavir to certain SYMTUZA interactions [see Drug Interactions (7)and Clinical Pharmacology (12.3)] .
5.5 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium aviuminfection, cytomegalovirus, Pneumocystis jiroveciipneumonia [PCP], or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, Guillain-Barré syndrome, and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable, and can occur many months after initiation of antiretroviral treatment.
5.6 New Onset or Worsening Renal Impairment
Postmarketing cases of renal impairment, including acute renal failure, proximal renal tubulopathy (PRT), and Fanconi syndrome have been reported with TAF-containing products; while most of these cases were characterized by potential confounders that may have contributed to the reported renal events, it is also possible these factors may have predisposed patients to tenofovir-related adverse events [see Adverse Reactions (6.1, 6.2)] . SYMTUZA is not recommended in patients with estimated creatinine clearance below 30 mL per minute.
Patients taking tenofovir prodrugs who have impaired renal function and those taking nephrotoxic agents including non-steroidal anti-inflammatory drugs are at increased risk of developing renal-related adverse reactions.
Prior to or when initiating SYMTUZA and during treatment with SYMTUZA, on a clinically appropriate schedule, assess serum creatinine, estimated creatinine clearance, urine glucose, and urine protein in all patients. In patients with chronic kidney disease, also assess serum phosphorus. Discontinue SYMTUZA in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome.
Cobicistat, a component of SYMTUZA, produces elevations of serum creatinine due to inhibition of tubular secretion of creatinine without affecting glomerular filtration. This effect should be considered when interpreting changes in estimated creatinine clearance in patients initiating SYMTUZA, particularly in patients with medical conditions or receiving drugs needing monitoring with estimated creatinine clearance. The elevation is typically seen within 2 weeks of starting therapy and is reversible after discontinuation. Patients who experience a confirmed increase in serum creatinine of greater than 0.4 mg/dL should be closely monitored for renal safety.
5.7 Sulfa Allergy
Darunavir contains a sulfonamide moiety. Monitor patients with a known sulfonamide allergy after initiating SYMTUZA. In clinical studies with darunavir co-administered with ritonavir, the incidence and severity of rash were similar in subjects with or without a history of sulfonamide allergy.
5.8 Lactic Acidosis/Severe Hepatomegaly with Steatosis
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with the use of nucleoside analogs, including emtricitabine, a component of SYMTUZA, and TDF, another prodrug of tenofovir, alone or in combination with other antiretrovirals. Treatment with SYMTUZA should be suspended in any patient who develops clinical or laboratory findings suggestive of lactic acidosis or pronounced hepatotoxicity (which may include hepatomegaly and steatosis even in the absence of marked transaminase elevations).
5.9 Diabetes Mellitus/Hyperglycemia
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV infected patients receiving HIV protease inhibitor (PI) therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued PI therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and causal relationships between HIV PI therapy and these events have not been established.
5.10 Fat Redistribution
Redistribution/accumulation of body fat, including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.11 Hemophilia
There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis in patients with hemophilia type A and B treated with HIV protease inhibitors (PIs). In some patients, additional factor VIII was given. In more than half of the reported cases, treatment with HIV PIs was continued or reintroduced if treatment had been discontinued. A causal relationship between PI therapy and these episodes has not been established.
-
6. ADVERSE REACTIONS
The following adverse reactions are discussed in other sections of the labeling:
- Severe acute exacerbations of hepatitis B [see Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- Severe skin reactions [see Warnings and Precautions (5.3)]
- Immune reconstitution syndrome [see Warnings and Precautions (5.5)]
- New onset or worsening renal impairment [see Warnings and Precautions (5.6)]
- Lactic acidosis/severe hepatomegaly with steatosis [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Clinical Trials in Adults
Adverse Reactions in Adults with No Prior Antiretroviral Treatment History
The safety profile of SYMTUZA in HIV-1 infected adults with no prior antiretroviral treatment history is based on Week 48 data from the AMBER trial, a randomized, double-blind, active-controlled trial where a total of 362 subjects received SYMTUZA once daily and 363 subjects received a combination of PREZCOBIX ®(fixed-dose combination of darunavir and cobicistat) and fixed-dose combination of emtricitabine and tenofovir disoproxil fumarate (FTC/TDF).
The proportion of subjects who discontinued treatment with SYMTUZA or PREZCOBIX+FTC/TDF due to adverse events, regardless of severity, were 2% and 4% respectively.
An overview of the most frequent (occurring in at least 2% of subjects) adverse reactions irrespective of severity reported in AMBER are presented in Table 1. An overview of the most frequent laboratory abnormalities of at least Grade 2 severity reported in AMBER are presented in Table 2. Changes from baseline in lipid parameters for patients receiving SYMTUZA and those receiving PREZCOBIX + FTC/TDF are presented in Table 3.
Most adverse reactions during treatment with SYMTUZA were grade 1 or 2 in severity. One grade 3 adverse reaction was reported and no grade 4 adverse reactions were reported during treatment with SYMTUZA.
Table 1: Adverse Reactions Reported in ≥2% of HIV-1 Infected Adults With No Prior Antiretroviral Treatment History in AMBER (Week 48 Analysis) SYMTUZA
(N=362)PREZCOBIX+FTC/TDF
(N=363)All Grades At least Grade 2 All Grades At least Grade 2 - *
- Includes pooled reported terms: dermatitis, dermatitis allergic, erythema, photosensitivity reaction, rash, rash generalized, rash macular, rash maculo-papular, rash morbilliform, rash pruritic, toxic skin eruption, urticaria
Diarrhea 9% 2% 11% 2% Rash * 8% 4% 7% 5% Nausea 6% 1% 10% 3% Fatigue 4% 1% 4% 1% Headache 3% 1% 2% 1% Abdominal discomfort 2% - 4% <1% Flatulence 2% <1% 1% - Adverse Reactions in Virologically-Suppressed Adults
The safety profile of SYMTUZA in virologically-suppressed HIV-1 infected adults is based on Week 48 data from 1,141 subjects in the EMERALD trial, a randomized, open-label, active-controlled trial where 763 subjects with a stable antiretroviral regimen consisting of a boosted protease inhibitor (bPI) [either darunavir once daily or atazanavir (both boosted with ritonavir or cobicistat), or lopinavir with ritonavir] combined with FTC/TDF switched to SYMTUZA, and 378 subjects who continued their treatment regimen of a bPI with FTC/TDF. Overall, the safety profile of SYMTUZA in subjects in this study was similar to that in subjects with no prior antiretroviral treatment history. The proportion of subjects who discontinued treatment with SYMTUZA due to adverse events, regardless of severity, was 1%.
Less Frequent Adverse Reactions
The following adverse reactions occurred in less than 2% of adults with no antiretroviral treatment history or virologically suppressed subjects receiving SYMTUZA, or are from studies described in the prescribing information of the individual component PREZISTA (darunavir).
Gastrointestinal Disorders: dyspepsia, pancreatitis (acute), vomiting
Skin and Subcutaneous Tissue Disorders: angioedema, pruritus, Stevens-Johnson syndrome
Metabolism and Nutrition Disorders: anorexia, diabetes mellitus, lipodystrophy
Reproductive System and Breast Disorders: gynecomastia
Musculoskeletal and Connective Tissue Disorders: myalgia, osteonecrosis
Psychiatric Disorders: abnormal dreams
Immune System Disorders: (drug) hypersensitivity, immune reconstitution inflammatory syndrome
Hepatobiliary Disorders: acute hepatitis
Laboratory Abnormalities
Table 2: Laboratory Abnormalities (Grade 2–4) Reported in ≥2% of Adults With No Prior Antiretroviral Treatment History in AMBER (Week 48 Analysis) Laboratory Parameter
GradeLimit SYMTUZA
N=362PREZCOBIX+FTC/TDF
N=363Creatinine Grade 2 >1.3 to 1.8 × ULN 4% 14% Grade 4 ≥3.5× ULN <1% 0 Triglycerides Grade 2 301–500 mg/dL 7% 4% Grade 3 501–1,000 mg/dL 1% 1% Grade 4 >1,000 mg/dL <1% <1% Total Cholesterol Grade 2 240–<300 mg/dL 17% 4% Grade 3 ≥ 300 mg/dL 2% 1% Low-Density Lipoprotein Cholesterol Grade 2 160–189 mg/dL 9% 4% Grade 3 ≥190 mg/dL 5% 1% Elevated Glucose Levels Grade 2 126–250 mg/dL 6% 6% Grade 3 251–500 mg/dL <1% 0 ALT and/or AST elevations (Grade 2–4 combined) occurred in 2% of adult subjects receiving SYMTUZA with no antiretroviral treatment history in AMBER (Week 48 Analysis). Results were consistent in subjects receiving PREZCOBIX+FTC/TDF.
Table 3: Lipid Values, Mean Change from Baseline, Reported in Adults With No Prior Antiretroviral Treatment History in AMBER (Week 48 Analysis) SYMTUZA
N=362PREZCOBIX+FTC/TDF
N=363Baseline Week 48 Baseline Week 48 Mean * mg/dL Change mg/dL Change N † N=304 ‡ N=290 - *
- The change from baseline is the mean of within-subject changes from baseline for subjects with both baseline and Week 48 values, or the last value carried forward prior to initiating lipid-lowering agent post-baseline.
- †
- N corresponds to the number of subjects with paired values and not on a lipid-lowering agent at screening/baseline. Subjects on lipid-lowering agents at screening/baseline were excluded from the analysis (6 out of 362 subjects on SYMTUZA, 8 out of 363 subjects on PREZCOBIX+FTC/TDF). Subjects initiating a lipid-lowering agent post-baseline had their last fasted on-treatment value (prior to starting the agent) carried forward (6 on SYMTUZA, 2 on PREZCOBIX+FTC/TDF).
- ‡
- One subject did not have a Week 48 result for LDL cholesterol (n=303).
Total cholesterol 168 +30 164 +11 HDL cholesterol 45 +6 44 +2 LDL cholesterol 100 +19 98 +5 Triglycerides 117 +34 112 +21 Total cholesterol to HDL ratio 4.1 0.2 4.0 0.1 The percentage of subjects starting any lipid lowering drug during treatment in the SYMTUZA and PREZCOBIX + FTC/TDF arm were 1.7% (n=6) and 0.6% (n=2), respectively.
Renal Laboratory Tests
In the AMBER trial, which enrolled 725 adults with no prior antiretroviral treatment history, subjects had a median baseline eGFR (estimated glomerular filtration rate) of 119 mL/min (SYMTUZA) and 118 mL/min (PREZCOBIX + FTC/TDF). From baseline to Week 48, mean (SD) serum creatinine increased by 0.05 (0.10) mg/dL in the SYMTUZA group and by 0.09 (0.11) mg/dL in the PREZCOBIX + FTC/TDF group. Median serum creatinine was 0.90 mg/dL (SYMTUZA) and 0.89 mg/dL (PREZCOBIX + FTC/TDF) at baseline and 0.95 mg/dL (SYMTUZA) and 0.97 mg/dL (PREZCOBIX +FTC/TDF) at Week 48. Increases in serum creatinine occurred by Week 2 of treatment and remained stable. Median urine protein-to-creatinine ratio (UPCR) was 47 mg/g (SYMTUZA) and 51 mg/g (PREZCOBIX + FTC/TDF) at baseline and 30 mg/g (SYMTUZA) and 34 mg/g (PREZCOBIX + FTC/TDF) at Week 48.
In the EMERALD trial which had 1,141 virologically-suppressed adults treated with an HIV protease inhibitor and TDF containing regimen with a median baseline eGFR of 104 mL/min (SYMTUZA) and 103 mL/min (bPI+FTC/TDF) who were randomized to continue their treatment regimen or switch to SYMTUZA, at Week 48, mean serum creatinine was similar to baseline for both those continuing baseline treatment and those switching to SYMTUZA. Mean (SD) serum creatinine was 0.98 (0.18) mg/dL (SYMTUZA) and 0.98 (0.19) mg/dL (bPI+FTC/TDF) at baseline and 0.99 (0.18) mg/dL (SYMTUZA) and 0.99 (0.21) mg/dL (bPI+FTC/TDF) at Week 48. Median serum creatinine was 0.97 mg/dL (SYMTUZA) and 0.98 mg/dL (bPI+FTC/TDF) at baseline and 1.0 mg/dL (SYMTUZA) and 0.97 mg/dL (bPI+FTC/TDF) at Week 48. Median UPCR was 62 mg/g (SYMTUZA) and 63 mg/g (bPI+FTC/TDF) at baseline and 37 mg/g (SYMTUZA) and 53 mg/g (bPI+FTC/TDF) at Week 48.
Bone Mineral Density
AMBER
The effects of SYMTUZA compared to PREZCOBIX + FTC/TDF on bone mineral density (BMD) change from baseline to Week 48 were assessed by dual-energy X-ray absorptiometry (DXA). The mean percentage change in BMD from baseline to Week 48 was −0.7% with SYMTUZA compared to −2.4% with PREZCOBIX + FTC/TDF at the lumbar spine and 0.2% compared to −2.7% at the total hip. BMD declines of 5% or greater at the lumbar spine were experienced by 16% of SYMTUZA subjects and 22% of PREZCOBIX + FTC/TDF subjects. BMD declines of 7% or greater at the femoral neck were experienced by 2% of SYMTUZA subjects and 15% of PREZCOBIX + FTC/TDF subjects. The long-term clinical significance of these BMD changes is not known.
EMERALD
In EMERALD, bPI and TDF-treated subjects were randomized to continue their TDF-based regimen or switch to SYMTUZA; changes in BMD from baseline to Week 48 were assessed by DXA. The mean percentage change in BMD from baseline to Week 48 was 1.5% with SYMTUZA compared to −0.6% with bPI + FTC/TDF at the lumbar spine and 1.4% compared to -0.3% at the total hip. BMD declines of 5% or greater at the lumbar spine were experienced by 2% of SYMTUZA subjects and 9% of bPI + FTC/TDF subjects. BMD declines of 7% or greater at the femoral neck were experienced by no SYMTUZA subjects and 2% of bPI + FTC/TDF subjects. The long-term clinical significance of these BMD changes is not known.
Clinical Trials in Pediatric Patients
Adverse Reactions in Pediatric Patients Weighing At Least 40 kg
No clinical trials with SYMTUZA were performed in pediatric patients. However, the safety of the components of SYMTUZA was evaluated in pediatric subjects of 12 to less than 18 years of age through clinical trials GS-US-216-0128 (virologically-suppressed, N=7 with weight ≥40 kg) for darunavir co-administered with cobicistat and other antiretroviral agents, and GS-US-292-0106 (treatment-naïve, N=50 with weight ≥35 kg) for a fixed-dose combination regimen containing cobicistat, emtricitabine, and tenofovir alafenamide together with elvitegravir. Safety analyses of the trials in these pediatric subjects did not identify new safety concerns compared to the known safety profile of SYMTUZA in adult subjects [see Clinical Studies (14.3)] .
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of darunavir. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Musculoskeletal and Connective Tissue Disorders:
Rhabdomyolysis (associated with co-administration with HMG-CoA reductase inhibitors)
Skin and Subcutaneous Tissue Disorders:
Toxic epidermal necrolysis, acute generalized exanthematous pustulosis, drug rash with eosinophilia and systemic symptoms [see Warnings and Precautions (5.3)]
Renal and Urinary Disorders:
Acute renal failure, acute tubular necrosis, proximal renal tubulopathy, Fanconi syndrome [see Warnings and Precautions (5.6)] , crystal nephropathy, and crystalluria
-
7. DRUG INTERACTIONS
7.1 Not Recommended With Other Antiretroviral Medications
SYMTUZA is a complete regimen for HIV-1 infection and co-administration with other antiretroviral medications for the treatment of HIV-1 infection is not recommended. For this reason, information regarding potential drug-drug interactions with other antiretroviral medications is not provided.
7.2 Potential for SYMTUZA to Affect Other Drugs
Darunavir co-administered with cobicistat is an inhibitor of CYP3A and CYP2D6. Cobicistat inhibits the following transporters: P-glycoprotein (P-gp), BCRP, MATE1, OATP1B1 and OATP1B3. Therefore, co-administration of SYMTUZA with drugs that are primarily metabolized by CYP3A and/or CYP2D6, or are substrates of P-gp, BCRP, MATE1, OATP1B1 or OATP1B3 may result in increased plasma concentrations of such drugs, which could increase or prolong their therapeutic effect and can be associated with adverse events. Co-administration of SYMTUZA with drugs that have active metabolite(s) formed by CYP3A may result in reduced plasma concentrations of these active metabolite(s), potentially leading to loss of their therapeutic effect (see Table 4).
7.3 Potential for Other Drugs to Affect SYMTUZA
Darunavir is metabolized by CYP3A. Cobicistat is metabolized by CYP3A and, to a minor extent, by CYP2D6. Co-administration of drugs that induce CYP3A activity are expected to increase the clearance of darunavir and cobicistat, resulting in lowered plasma concentrations which may lead to loss of therapeutic effect and development of resistance. Co-administration of SYMTUZA with other drugs that inhibit CYP3A may result in increased plasma concentrations of darunavir and cobicistat (see Table 4).
Tenofovir alafenamide (TAF) is a substrate of P-gp, BCRP, OATP1B1, and OATP1B3. Drugs that strongly affect P-gp activity may lead to changes in TAF absorption. Drugs that induce P-gp activity are expected to decrease the absorption of TAF, resulting in decreased plasma concentrations of TAF, which may lead to loss of therapeutic effect of SYMTUZA and development of resistance. Co-administration of SYMTUZA with other drugs that inhibit P-gp may increase the absorption and plasma concentrations of TAF (see Table 4).
7.4 Drugs Affecting Renal Function
Because emtricitabine and tenofovir are primarily excreted by the kidneys through glomerular filtration and active tubular secretion, co-administration of SYMTUZA with drugs that reduce renal function or compete for active tubular secretion may increase concentrations of emtricitabine, tenofovir, and other renally eliminated drugs and this may increase the risk of adverse reactions. Some examples of drugs that are eliminated by active tubular secretion include, but are not limited to, acyclovir, cidofovir, ganciclovir, valacyclovir, valganciclovir, aminoglycosides (e.g., gentamicin), and high-dose or multiple NSAIDs [see Warnings and Precautions (5.6)] .
7.5 Significant Drug Interactions
Table 4 provides examples of established or potentially clinically significant drug interactions with SYMTUZA and recommended steps to prevent or manage these interactions. These recommendations are based on drug interaction trials conducted with the components of SYMTUZA, as individual agents or in combination, or are predicted interactions. No drug interaction trials have been performed with SYMTUZA or with all the components administered together. Drug interaction trials have been conducted with darunavir co-administered with ritonavir or cobicistat or with emtricitabine and tenofovir prodrugs. The table includes potentially significant interactions but is not all inclusive ,and therefore the label of each drug that is co- administered with SYMTUZA should be consulted for information related to the route of metabolism, interaction pathways, potential risks, and specific actions to be taken with regard to co- administration.
Table 4: Significant Drug Interactions Concomitant Drug Class: Drug Name
ExamplesEffect on Concentration Clinical Comment This table is not all inclusive
↑ = increase, ↓ = decrease, ↔ = no effectAlpha 1-adrenoreceptor antagonist:
alfuzosin↑ alfuzosin Co-administration is contraindicated due to potential for serious and/or life-threatening reactions such as hypotension. Antibacterials:
clarithromycin, erythromycin, telithromycin↑ darunavir
↑ cobicistat
↑ antibacterialConsider alternative antibiotics with concomitant use of SYMTUZA. Anticancer agents:
dasatinib, nilotinib↑ anticancer agent A decrease in the dosage or an adjustment of the dosing interval of dasatinib or nilotinib may be necessary when co-administered with SYMTUZA. Consult the dasatinib and nilotinib prescribing information for dosing instructions. vinblastine, vincristine For vincristine and vinblastine, consider temporarily withholding the cobicistat-containing antiretroviral regimen in patients who develop significant hematologic or gastrointestinal side effects when SYMTUZA is administered concurrently with vincristine or vinblastine. If the antiretroviral regimen must be withheld for a prolonged period, consider initiating a revised regimen that does not include a CYP3A or P-gp inhibitor. Anticoagulants:
Direct Oral Anticoagulants (DOACs)
apixaban↑ apixaban Due to potentially increased bleeding risk, dosing recommendations for co-administration of apixaban with SYMTUZA depend on the apixaban dose. Refer to apixaban dosing instructions for co-administration with P-gp and strong CYP3A inhibitors in apixaban prescribing information. rivaroxaban ↑ rivaroxaban Co-administration of rivaroxaban with SYMTUZA is not recommended because it may lead to an increased bleeding risk. dabigatran etexilate
edoxaban↑ dabigatran
↑ edoxaban
Refer to the dabigatran etexilate or edoxaban prescribing information for recommendations regarding co-administration. The specific recommendations are based on indication, renal function, and effect of the co-administered P-gp inhibitors on the concentration of dabigatran or edoxaban. Clinical monitoring is recommended when a DOAC not affected by CYP3A4 but transported by P-gp, including dabigatran etexilate and edoxaban, is co-administered with SYMTUZA. Other Anticoagulants warfarin warfarin: effect unknown Monitor international normalized ratio (INR) upon co-administration of SYMTUZA with warfarin. Anticonvulsants:
carbamazepine, phenobarbital, phenytoin↓ cobicistat
↓ darunavir
↓ tenofovir alafenamideCo-administration is contraindicated due to potential for loss of therapeutic effect and development of resistance. Anticonvulsants with CYP3A induction effects that are NOT contraindicated:
e.g., eslicarbazepine, oxcarbazepine↓ cobicistat
↓ tenofovir alafenamide darunavir: effect unknownConsider alternative anticonvulsant or antiretroviral therapy to avoid potential changes in exposures. If co-administration is necessary, monitor for lack or loss of virologic response. Anticonvulsants that are metabolized by CYP3A:
e.g., clonazepam↑ clonazepam Clinical monitoring of anticonvulsants is recommended. Antidepressants:
Selective Serotonin Reuptake Inhibitors (SSRIs):
e.g., paroxetine, sertralineSSRIs: effects unknown When co-administering with SSRIs, TCAs, or trazodone, careful dose titration of the antidepressant to the desired effect, including using the lowest feasible initial or maintenance dose, and monitoring for antidepressant response are recommended. Tricyclic Antidepressants (TCAs):
e.g., amitriptyline, desipramine, imipramine, nortriptyline↑ TCAs Other antidepressants:
trazodone↑ trazodone Antifungals:
itraconazole, isavuconazole, ketoconazole, posaconazole↑ darunavir
↑ cobicistatMonitor for increased darunavir or cobicistat and/or antifungal adverse reactions. ↑ itraconazole
↑ isavuconazole
↑ ketoconazole
↔ posaconazole (not studied)Specific dosing recommendations are not available for co-administration with these antifungals. Monitor for increased itraconazole or ketoconazole adverse reactions. voriconazole voriconazole: effects unknown Co-administration with voriconazole is not recommended unless benefit/risk assessment justifies the use of voriconazole. Anti-gout:
colchicine↑ colchicine Co-administration is contraindicated in patients with renal and/or hepatic impairment due to potential for serious and/or life-threatening reactions.
For patients without renal or hepatic impairment:- Treatment of gout flares – co-administration of colchicine:0.6 mg (1 tablet) ×1 dose, followed by 0.3 mg (half tablet) 1 hour later. Treatment course to be repeated no earlier than 3 days.
- Prophylaxis of gout flares – co-administration of colchicine:If the original regimen was 0.6 mg twice a day, the regimen should be adjusted to 0.3 mg once a day. If the original regimen was 0.6 mg once a day, the regimen should be adjusted to 0.3 mg once every other day.
- Treatment of familial Mediterranean fever – co-administration of colchicine:Maximum daily dose of 0.6 mg (may be given as 0.3 mg twice a day).
Antimalarial:
artemether/lumefantrineartemether: effect unknown
lumefantrine: effect unknownMonitor for a potential decrease of antimalarial efficacy or potential QT prolongation. Antimycobacterials:
rifampin↓ cobicistat
↓ darunavir
↓ tenofovir alafenamideCo-administration is contraindicated due to potential for loss of therapeutic effect and development of resistance. rifabutin ↑ rifabutin
↓ TAF
cobicistat: effects unknown
darunavir: effects unknownCo-administration of SYMTUZA with rifabutin is not recommended. If the combination is needed, the recommended dose of rifabutin is 150 mg every other day. Monitor for rifabutin-associated adverse reactions including neutropenia and uveitis. rifapentine ↓ darunavir
↓ TAFCo-administration with rifapentine is not recommended. Antipsychotics:
lurasidone↑ lurasidone Co-administration is contraindicated due to potential for serious and/or life-threatening reactions. pimozide ↑ pimozide Co-administration is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias. e.g., perphenazine, risperidone, thioridazine ↑ antipsychotic A decrease in the dose of antipsychotics that are metabolized by CYP3A or CYP2D6 may be needed when co-administered with SYMTUZA. quetiapine ↑ quetiapine Initiation of SYMTUZA in patients taking quetiapine:Consider alternative antiretroviral therapy to avoid increases in quetiapine exposure. If co-administration is necessary, reduce the quetiapine dose to 1/6 of the current dose and monitor for quetiapine-associated adverse reactions. Refer to the quetiapine prescribing information for recommendations on adverse reaction monitoring.
Initiation of quetiapine in patients taking SYMTUZA:Refer to the quetiapine prescribing information for initial dosing and titration of quetiapine.β-Blockers:
e.g., carvedilol, metoprolol, timolol↑ beta-blockers Clinical monitoring is recommended for co-administration with beta-blockers that are metabolized by CYP2D6. Calcium channel blockers:
e.g., amlodipine, diltiazem, felodipine, nifedipine, verapamil↑ calcium channel blockers Clinical monitoring is recommended for co-administration with calcium channel blockers metabolized by CYP3A. Cardiac Disorders: ranolazine, ivabradine ↑ ranolazine Co-administration is contraindicated due to potential for serious and/or life-threatening reactions. dronedarone ↑ dronedarone Co-administration is contraindicated due to potential for serious and/or life-threatening reactions such as cardiac arrhythmias Other antiarrhythmics
e.g., amiodarone, disopyramide, flecainide, lidocaine (systemic), mexiletine, propafenone, quinidine↑ antiarrhythmics Clinical monitoring is recommended upon co-administration with antiarrhythmics. digoxin ↑ digoxin When co-administering with digoxin, titrate the digoxin dose and monitor digoxin concentrations. Corticosteroids:
dexamethasone (systemic)↓ darunavir
↓ cobicistat
Co-administration with systemic dexamethasone or other systemic corticosteroids that induce CYP3A may result in loss of therapeutic effect and development of resistance to SYMTUZA. Consider alternative corticosteroids. Corticosteroids primarily metabolized by CYP3A:
e.g.,
betamethasone
budesonide
ciclesonide
fluticasone
methylprednisolone
mometasone
triamcinolone↑ corticosteroids Co-administration with corticosteroids (all routes of administration) of which exposures are significantly increased by strong CYP3A inhibitors can increase the risk for Cushing's syndrome and adrenal suppression.
Alternative corticosteroids including beclomethasone, prednisone, and prednisolone (for which PK and/or PD are less affected by strong CYP3A inhibitors relative to other steroids) should be considered, particularly for long term use.Endothelin receptor antagonists:
bosentan↓ darunavir
↓ cobicistat
↑ bosentanInitiation of bosentan in patients taking SYMTUZA: In patients who have been receiving SYMTUZA for at least 10 days, start bosentan at 62.5 mg once daily or every other day based upon individual tolerability.
Initiation of SYMTUZA in patients on bosentan:Discontinue use of bosentan at least 36 hours prior to initiation of SYMTUZA. After at least 10 days following the initiation of SYMTUZA, resume bosentan at 62.5 mg once daily or every other day based upon individual tolerability.
Switching from darunavir co-administered with ritonavir to SYMTUZA in patients on bosentan:Maintain bosentan dose.Ergot derivatives: e.g., dihydroergotamine, ergotamine, methylergonovine ↑ ergot derivatives Co-administration is contraindicated due to potential for serious and/or life-threatening reactions such as acute ergot toxicity characterized by peripheral vasospasm and ischemia of the extremities and other tissues. Hepatitis C virus (HCV):
Direct-Acting Antivirals:elbasvir/grazoprevir ↑ elbasvir/grazoprevir Co-administration is contraindicated due to potential for the increased risk of alanine transaminase (ALT) elevations. glecaprevir/pibrentasvir ↑ glecaprevir
↑ pibrentasvirCo-administration of SYTMUZA with glecaprevir/pibrentasvir is not recommended. Herbal product: St. John's wort ( Hypericum perforatum) ↓ cobicistat
↓ darunavir
↓ tenofovir alafenamideCo-administration is contraindicated due to potential for loss of therapeutic effect and development of resistance. Hormonal contraceptives: Additional or alternative (non-hormonal) forms of contraception should be considered when estrogen based contraceptives are co-administered with SYMTUZA. drosperinone/ethinylestradiol ↑ drosperinone
↓ ethinylestradiolFor co-administration with drospirenone, clinical monitoring is recommended due to the potential for hyperkalemia. other progestin/estrogen contraceptives progestin: effects unknown
estrogen: effects unknownNo data are available to make recommendations on co-administration with oral or other hormonal contraceptives. Immunosuppressants:
cyclosporine, sirolimus, tacrolimus↑ immunosuppressants These immunosuppressant agents are metabolized by CYP3A. Therapeutic drug monitoring is recommended with concomitant use. Immunosuppressant /neoplastic:
everolimusCo-administration of everolimus and SYMTUZA is not recommended. irinotecan Discontinue SYMTUZA at least 1 week prior to starting irinotecan therapy. Do not administer SYMTUZA with irinotecan unless there are no therapeutic alternatives. Inhaled beta agonist: salmeterol ↑ salmeterol Co-administration with salmeterol is not recommended and may result in increased risk of cardiovascular adverse events associated with salmeterol, including QT prolongation, palpitations, and sinus tachycardia. Lipid modifying agents:
HMG-CoA reductase inhibitors:lovastatin, simvastatin ↑ lovastatin
↑ simvastatinCo-administration is contraindicated due to potential for serious reactions such as myopathy including rhabdomyolysis. e.g., atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin ↑ atorvastatin
↑ fluvastatin
↑ pravastatin
↑ rosuvastatin
pitavastatin: effect unknownFor atorvastatin, fluvastatin, pitavastatin, pravastatin, and rosuvastatin, start with the lowest recommended dose and titrate while monitoring for safety. Dosage recommendations with atorvastatin or rosuvastatin are as follows: - atorvastatin dosage should not exceed 20 mg/day
- rosuvastatin dosage should not exceed 20 mg/day
Other lipid modifying agents:
lomitapide↑ lomitapide Co-administration is contraindicated due to potential for markedly increased transaminases associated with increased plasma concentrations of lomitapide. Narcotic analgesics metabolized by CYP3A:
e.g., fentanyl, oxycodone↑ fentanyl
↑ oxycodoneCareful monitoring of therapeutic effects and adverse reactions associated with CYP3A-metabolized narcotic analgesics (including potentially fatal respiratory depression) is recommended with co-administration. tramadol ↑ tramadol A dose decrease may be needed for tramadol with concomitant use. Narcotic analgesic for treatment of opioid dependence:
buprenorphine, buprenorphine/naloxone, methadonebuprenorphine or buprenorphine/ naloxone: effects unknown
methadone: effects unknownInitiation of buprenorphine, buprenorphine/naloxone or methadone in patients taking SYMTUZA:Carefully titrate the dose of buprenorphine, buprenorphine/naloxone or methadone to the desired effect; use the lowest feasible initial or maintenance dose.
Initiation of SYMTUZA in patients taking buprenorphine, buprenorphine/naloxone, or methadone:A dose adjustment for buprenorphine, buprenorphine/naloxone, or methadone may be needed. Monitor clinical signs and symptoms.Opioid Antagonist naloxegol ↑ naloxegol Co-administration of SYMTUZA and naloxegol is contraindicated due to potential for precipitating opioid withdrawal symptoms. Phosphodiesterase PDE-5 inhibitors: ↑ PDE-5 inhibitors e.g., avanafil, sildenafil, tadalafil, vardenafil Co-administration with avanafil is not recommended because a safe and effective avanafil dosage regimen has not been established.
Co-administration with PDE-5 inhibitors may result in an increase in PDE-5 inhibitor-associated adverse reactions including hypotension, syncope, visual disturbances, and priapism.
Use of PDE-5 inhibitors for pulmonary arterial hypertension (PAH):
Co-administration with sildenafil used for PAH is contraindicated due to potential for sildenafil associated adverse reactions (which include visual disturbances, hypotension, prolonged erection, and syncope). The following dose adjustments are recommended for use of tadalafil with SYMTUZA:- Initiation of tadalafil in patients taking SYMTUZA:In patients receiving SYMTUZA for at least one week, start tadalafil at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability.
- Initiation of SYMTUZA in patients taking tadalafil:Avoid use of tadalafil during the initiation of SYMTUZA. Stop tadalafil at least 24 hours prior to starting SYMTUZA. After at least one week following the initiation of SYMTUZA, resume tadalafil at 20 mg once daily. Increase to 40 mg once daily based upon individual tolerability.
- Patients switching from darunavir co-administered with ritonavir to SYMTUZA:Maintain tadalafil dose.
Sildenafil at a single dose not exceeding 25 mg in 48 hours, vardenafil at a single dose not exceeding 2.5 mg dose in 72 hours, or tadalafil at a single dose not exceeding 10 mg dose in 72 hours can be used with increased monitoring for PDE-5 inhibitor-associated adverse reactions.Platelet aggregation inhibitor:
ticagrelor↑ticagrelor Co-administration of SYMTUZA and ticagrelor is not recommended. clopidogrel ↓ clopidogrel active metabolite Co-administration of SYMTUZA and clopidogrel is not recommended due to potential reduction of the antiplatelet activity of clopidogrel. prasugrel ↔ prasugrel active metabolite No dose adjustment is needed when prasugrel is co-administered with SYMTUZA. Sedatives/hypnotics:
orally administered midazolam, triazolam↑ midazolam
↑ triazolamCo-administration is contraindicated due to potential for serious and/or life-threatening reactions such as prolonged or increased sedation or respiratory depression. metabolized by CYP3A:
e.g., buspirone, diazepam, estazolam, zolpidem↑ sedatives/hypnotics With concomitant use, titration is recommended with sedatives/hypnotics metabolized by CYP3A and a lower dose of the sedatives/hypnotics should be considered with monitoring for increased and prolonged effects or adverse reactions. parenterally administered midazolam Co-administration of parenteral midazolam should be done in a setting that ensures close clinical monitoring and appropriate medical management in case of respiratory depression and/or prolonged sedation. Dose reduction for parenteral midazolam should be considered, especially if more than a single dose of midazolam is administered. Urinary antispasmodics fesoterodine ↑ fesoterodine When fesoterodine is co-administered with SYMTUZA, do not exceed a fesoterodine dose of 4 mg once daily. solifenacin ↑ solifenacin When solifenacin is co-administered with SYMTUZA, do not exceed a solifenacin dose of 5 mg once daily. -
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to SYMTUZA during pregnancy. Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry (APR) at 1-800-258-4263.
Risk Summary
SYMTUZA is not recommended during pregnancy because of substantially lower exposures of darunavir and cobicistat during the second and third trimesters [see Dosage and Administration (2.5)] . A study evaluating the pharmacokinetics of antiretrovirals during pregnancy demonstrated substantially lower exposures of darunavir and cobicistat in the second and third trimesters compared to the post-partum period (see Data) and [see Clinical Pharmacology (12.3)].
Prospective pregnancy data from the APR are not sufficient to adequately assess the risk of birth defects or miscarriage. However, available data from the APR show no statistically significant difference in the overall risk of major birth defects for darunavir, cobicistat, emtricitabine, or tenofovir alafenamide (TAF) compared with the background rate for major birth defects of 2.7% in a U.S. reference population of the Metropolitan Atlanta Congenital Defects Program (MACDP) (see Data) . The rate of miscarriage is not reported in the APR. The estimated background rate of miscarriage in clinically recognized pregnancies in the U.S. general population is 15–20%. The background risk of major birth defects and miscarriage for the indicated population is unknown.
In animal reproduction studies, no adverse developmental effects were observed when the components of SYMTUZA were administered separately at darunavir exposures less than 1- (mice and rabbits) and 2.6-times (rats) higher, at cobicistat exposures 1.7- and 4.1-times higher (rats and rabbits respectively), at emtricitabine exposures 88- and 7.3- times higher (mice and rabbits, respectively), and tenofovir alafenamide exposures equal to or 85- times higher (rats and rabbits, respectively) than human exposures at the recommended daily dose of these components in SYMTUZA (see Data) . No adverse developmental effects were seen when cobicistat was administered to rats through lactation at cobicistat exposures up to 1.1 times the human exposure at the recommended therapeutic dose.
Clinical Considerations
Not Recommended During Pregnancy
SYMTUZA is not recommended for use during pregnancy because of substantially lower exposures of darunavir and cobicistat during pregnancy (see Data) and [see Clinical Pharmacology (12.3)] .
SYMTUZA should not be initiated in pregnant individuals. An alternative regimen is recommended for individuals who become pregnant during therapy with SYMTUZA.
Data
Human Data
Darunavir and cobicistat in combination with a background regimen was evaluated in a clinical trial of 7 pregnant individuals taking darunavir and cobicistat prior to enrollment and who were willing to remain on darunavir and cobicistat throughout the study. The study period included the second and third trimesters, and through 12 weeks postpartum. Six pregnant individuals completed the trial.
Exposure to darunavir and cobicistat as part of an antiretroviral regimen was substantially lower during the second and third trimesters of pregnancy compared with postpartum [see Clinical Pharmacology (12.3)] .
One out of 6 pregnant individuals who completed the study experienced virologic failure with HIV-1 RNA >1,000 copies/mL from the third trimester visit through the postpartum period. Five pregnant individuals had sustained virologic response (HIV RNA <50 copies/mL) throughout the study period. There are no clinical data on the virologic response when darunavir and cobicistat are initiated during pregnancy.
Prospective reports from the APR of overall major birth defects in pregnancies exposed to the components of SYMTUZA are compared with a U.S. background major birth defect rate. Methodological limitations of the APR include the use of MACDP as the external comparator group. Limitations of using an external comparator include differences in methodology and populations, as well as confounding due to the underlying disease.
Darunavir: Based on prospective reports to the APR of over 960 exposures to darunavir-containing regimens during pregnancy resulting in live births (including over 640 exposed in the first trimester and over 320 exposed in the second/third trimester), the prevalence of birth defects in live births was 3.7% (95% CI: 2.4% to 5.5%) with first trimester exposure to darunavir containing-regimens and 2.5% (95% CI: 1.1% to 4.9%) with second/third trimester exposure to darunavir-containing regimens.
Cobicistat: Based on prospective reports to the APR of over 560 exposures to cobicistat-containing regimens during pregnancy resulting in live births (including over 470 exposed in the first trimester and over 80 exposed in the second/third trimester), the prevalence of birth defects in live births was 3.6% (95% CI: 2.1% to 5.7%) and 1.1% (95% CI: 0.0% to 6.2%) with first and second/third trimester, respectively, to cobicistat-containing regimens.
Emtricitabine: Based on prospective reports to the APR of over 5400 exposures to emtricitabine-containing regimens during pregnancy resulting in live births (including over 3900 exposed in the first trimester and over 1500 exposed in the second/third trimester), the prevalence of birth defects in live births was 2.6% (95% CI: 2.2% to 3.2%) with first trimester exposure to emtricitabine-containing regimens and 2.7% (95% CI: 1.9% to 3.7%) with the second/third trimester exposure to emtricitabine-containing regimens.
Tenofovir alafenamide (TAF): Based on prospective reports to the APR of over 660 exposures to TAF-containing regimens during pregnancy resulting in live births (including over 520 exposed in the first trimester and over 130 exposed in the second/third trimester), the prevalence of birth defects in live births was 4.2% (95% CI: 2.6% to 6.3%) and 3.0% (95% CI: 0.8% to 7.5%) with first and second/third trimester exposure, respectively, to TAF-containing regimens.
Animal Data
Darunavir: Reproduction studies conducted with darunavir showed no embryotoxicity or teratogenicity in mice (doses up to 1000 mg/kg from gestation day (GD) 6–15 with darunavir alone) and rats (doses up to 1000 mg/kg from GD 7–19 in the presence or absence of ritonavir) as well as in rabbits (doses up to 1000 mg/kg/day from GD 8–20 with darunavir alone). In these studies, darunavir exposures (based on AUC) were higher in rats (2.6-fold), whereas in mice and rabbits, exposures were lower (less than 1-fold) compared to those obtained in humans at the recommended daily dose of darunavir in SYMTUZA.
Cobicistat: Cobicistat was administered orally to pregnant rats at doses up to 125 mg/kg/day on GD 6–17. Increases in post-implantation loss and decreased fetal weights were observed at a maternal toxic dose of 125 mg/kg/day. No malformations were noted at doses up to 125 mg/kg/day. Systemic exposures (AUC) at 50 mg/kg/day in pregnant females were 1.7 times higher than human exposures at the recommended daily dose of cobicistat in SYMTUZA.
In pregnant rabbits, cobicistat was administered orally at doses up to 100 mg/kg/day during GD 7–20. No maternal or embryo/fetal effects were noted at the highest dose of 100 mg/kg/day. Systemic exposures (AUC) at 100 mg/kg/day were 4.1 times higher than human exposures at the recommended daily dose of cobicistat in SYMTUZA.
In a pre/postnatal developmental study in rats, cobicistat was administered orally at doses up to 75 mg/kg from GD 6 to postnatal day 20, 21, or 22. At doses of 75 mg/kg/day, neither maternal nor developmental toxicity was noted. Systemic exposures (AUC) at this dose were 1.1 times the human exposures at the recommended daily dose of cobicistat in SYMTUZA.
Emtricitabine: Emtricitabine was administered orally to pregnant mice and rabbits (up to 1000 mg/kg/day) through organogenesis (on GD 6 through 15, and 7 through 19, respectively). No significant toxicological effects were observed in embryo-fetal toxicity studies performed with emtricitabine in mice at exposures approximately 88 times higher and in rabbits approximately 7.3 times higher than human exposures at the recommended daily dose of emtricitabine in SYMTUZA.
In a pre/postnatal development study, mice were administered doses up to 1000 mg/kg/day; no significant adverse effects directly related to drug were observed in the offspring exposed daily from before birth ( in utero) through sexual maturity at daily exposures of approximately 88 times higher than human exposures at the recommended daily dose of emtricitabine in SYMTUZA.
Tenofovir Alafenamide (TAF): TAF was administered orally to pregnant rats (up to 250 mg/kg/day) and rabbits (up to 100 mg/kg/day) through organogenesis (on GD 6 through 17, and 7 through 20, respectively). No adverse embryo-fetal effects were observed in rats and rabbits at TAF exposures approximately similar to (rats) and 85 times higher (rabbits) than the exposure in humans at the recommended daily dose. TAF is rapidly converted to tenofovir; the observed tenofovir exposure in rats and rabbits were 51 (rats) and 80 (rabbits) times higher than human tenofovir exposures at the recommended daily dose of TAF in SYMTUZA.
Since TAF is rapidly converted to tenofovir and a lower tenofovir exposure in rats and mice was observed after TAF administration compared to TDF (another prodrug of tenofovir) administration, a pre/postnatal development study in rats was conducted only with TDF. Doses up to 600 mg/kg/day were administered through lactation; no adverse effects were observed in the offspring on GD 7 [and lactation day 20] at tenofovir exposures of approximately 14 [21] times higher than the exposure in humans at the recommended daily dose of TDF.
8.2 Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that HIV-infected mothers in the United States must not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection.
Based on published data, emtricitabine has been shown to be present in human breast milk. There are no data on the presence of darunavir, cobicistat, or TAF in human milk, the effects on the breastfed infant, or the effects on milk production. Darunavir and cobicistat are present in the milk of lactating rats. Tenofovir has been shown to be present in the milk of lactating rats and rhesus monkeys after administration of TDF (see Data) . Because of the potential for (1) HIV transmission (in HIV-negative infants), (2) developing viral resistance (in HIV-positive infants), and (3) serious adverse reactions in breastfed infants, instruct mothers not to breastfeed if they are receiving SYMTUZA.
Data
Animal Data
Darunavir: Studies in rats (with darunavir alone or with ritonavir) have demonstrated that darunavir is excreted in milk. In the rat pre- and post-natal development study, a reduction in pup body weight gain was observed due to exposure of pups to drug substances via milk. The maximal maternal plasma exposures achieved with darunavir (up to 1000 mg/kg with ritonavir) were approximately 66% of those obtained in humans at the recommended clinical dose of darunavir with ritonavir.
Cobicistat: During the pre/postnatal developmental toxicology study, at doses up to 75 mg/kg/day, mean cobicistat milk to plasma ratio of up to 1.9 was measured 2 hours after administration to rats on lactation day 10.
Tenofovir Alafenamide: Studies in rats and monkeys have demonstrated that tenofovir is excreted in milk. Tenofovir was excreted into the milk of lactating rats following oral administration of TDF (up to 600 mg/kg/day) at up to approximately 24% of the median plasma concentration in the highest dosed animals at lactation day 11. Tenofovir was excreted into the milk of lactating rhesus monkeys, following a single subcutaneous (30 mg/kg) dose of tenofovir at concentrations up to approximately 4% of plasma concentration resulting in exposure (AUC) of approximately 20% of plasma exposure.
8.4 Pediatric Use
The safety and effectiveness of SYMTUZA for the treatment of HIV-1 infection in pediatric patients weighing at least 40 kg was established through studies with components of SYMTUZA. Use of SYMTUZA in this group is supported by evidence from adequate and well-controlled studies of SYMTUZA in adults with additional pharmacokinetic, safety, and virologic data from studies of components of SYMTUZA (Trials GS-US-216-0128 and GS-US-292-0106) in pediatric subjects with HIV-1 infection aged 12 to less than 18 years [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.3)] .
The safety and effectiveness of SYMTUZA have not been established in pediatric patients weighing less than 40 kg.
Darunavir, a component of SYMTUZA is not recommended in pediatric patients below 3 years of age because of toxicity and mortality observed in juvenile rats dosed with darunavir.
Juvenile Animal Toxicity Data
Darunavir: In a juvenile toxicity study where rats were directly dosed with darunavir (up to 1000 mg/kg), deaths occurred from post-natal day 5 at plasma exposure levels ranging from 0.1 to 1.0 of the human exposure levels. In a 4-week rat toxicology study, when dosing was initiated on post-natal day 23 (the human equivalent of 2 to 3 years of age), no deaths were observed with a plasma exposure (in combination with ritonavir) 2 times the human plasma exposure levels.
8.5 Geriatric Use
Clinical trials of SYMTUZA included 35 subjects aged above 65 years of which 26 received SYMTUZA. No differences in safety or efficacy have been observed between elderly subjects and those aged 65 years or less. In general, caution should be exercised in the administration and monitoring of SYMTUZA in elderly patients, reflecting the greater frequency of decreased hepatic function and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)] .
8.6 Renal Impairment
SYMTUZA is not recommended in patients with severe renal impairment (creatinine clearance below 30 mL per minute). No dosage adjustment of SYMTUZA is required in patients with creatinine clearance greater than or equal to 30 mL per minute [see Clinical Pharmacology (12.3)] .
Cobicistat has been shown to decrease creatinine clearance without affecting actual renal glomerular function. Dosing recommendations are not available for drugs that require dosage adjustment for renal impairment when used in combination with SYMTUZA [see Warnings and Precautions (5.6)] .
8.7 Hepatic Impairment
No dosage adjustment of SYMTUZA is required in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. SYMTUZA has not been studied in patients with severe hepatic impairment (Child-Pugh Class C) and there are only limited data regarding the use of SYMTUZA components in this population. Therefore, SYMTUZA is not recommended for use in patients with severe hepatic impairment [see Clinical Pharmacology (12.3)] .
-
10. OVERDOSAGE
Human experience of acute overdose with SYMTUZA is limited. There is no specific antidote for overdose with SYMTUZA. Treatment of overdose with SYMTUZA consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
Since darunavir and cobicistat are highly bound to plasma proteins, it is unlikely that they will be significantly removed by hemodialysis or peritoneal dialysis. Hemodialysis treatment removes approximately 30% of the emtricitabine dose over a 3-hour dialysis period starting within 1.5 hours of emtricitabine dosing (blood flow rate of 400 mL/min and a dialysate flow rate of 600 mL/min). Tenofovir is efficiently removed by hemodialysis with an extraction coefficient of approximately 54%. It is not known whether emtricitabine or tenofovir can be removed by peritoneal dialysis.
-
11. DESCRIPTION
SYMTUZA ®(darunavir, cobicistat, emtricitabine, and tenofovir alafenamide) is a fixed-dose combination tablet.
- Darunavir is an inhibitor of the HIV-1 protease.
- Cobicistat is a mechanism-based inhibitor of cytochrome P450 (CYP) enzymes of the CYP3A family.
- Emtricitabine, a synthetic nucleoside analog of cytidine, is an HIV nucleoside analog reverse transcriptase inhibitor (HIV NRTI).
- Tenofovir alafenamide, an HIV NRTI, is converted in vivoto tenofovir, an acyclic nucleoside phosphonate (nucleotide) analog of adenosine 5′-monophosphate.
SYMTUZA tablets are for oral administration. Each tablet contains darunavir ethanolate equivalent to 800 mg of darunavir, 150 mg of cobicistat, 200 mg of emtricitabine, and 11.2 mg of tenofovir alafenamide fumarate equivalent to 10 mg of tenofovir alafenamide. The tablets include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, and microcrystalline cellulose. The tablets are film-coated with a coating material containing polyethylene glycol (macrogol), polyvinyl alcohol (partially hydrolyzed), talc, titanium dioxide, and yellow ferric oxide.
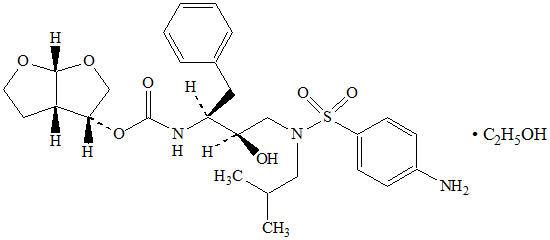
Darunavir: Darunavir, in the form of darunavir ethanolate, has the following chemical name: [(1 S,2 R)-3-[[(4-aminophenyl)sulfonyl](2-methylpropyl)amino]-2-hydroxy-1-(phenylmethyl)propyl]-carbamic acid (3 R,3a S,6a R)-hexahydrofuro[2,3- b]furan-3-yl ester monoethanolate. Its molecular formula is C 27H 37N 3O 7S ∙ C 2H 5OH and its molecular weight is 593.73. Darunavir ethanolate has the following structural formula:
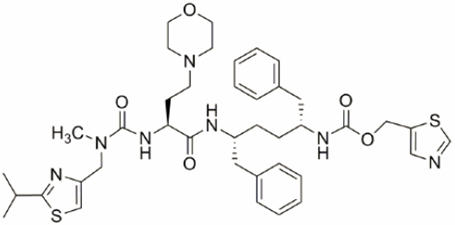
Cobicistat: Cobicistat is adsorbed onto silicon dioxide. The chemical name for cobicistat is 1,3-thiazol-5-ylmethyl[(2 R,5 R)-5-{[(2 S)-2-[(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)amino]-4-(morpholin-4yl)butanoyl]amino}-1,6-diphenylhexan-2-yl]carbamate. It has a molecular formula of C 40H 53N 7O 5S 2and a molecular weight of 776.02. It has the following structural formula:
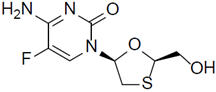
Emtricitabine: The chemical name of emtricitabine is 4-amino-5-fluoro-1-(2 R-hydroxymethyl-[1,3]-oxathiolan-5 S-yl)-(1H)-pyrimidin-2-one. Emtricitabine is the (-)enantiomer of a thio analog of cytidine, which differs from other cytidine analogs in that it has a fluorine in the 5 position. Emtricitabine has a molecular formula of C 8H 10FN 3O 3S and a molecular weight of 247.24. It has the following structural formula:
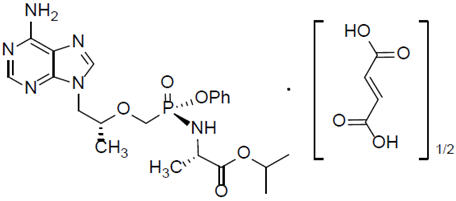
Tenofovir alafenamide: The chemical name of tenofovir alafenamide fumarate drug substance is L-alanine, N-[( S)-[[(1 R)-2-(6-amino-9 H-purin-9-yl)-1-methylethoxy]methyl]phenoxyphosphinyl]-,1-methylethyl ester, (2 E)-2-butenedioate (2:1). Tenofovir alafenamide fumarate has a molecular formula of C 21H 29O 5N 6P∙½(C 4H 4O 4) and a formula weight of 534.50. It has the following structural formula:
-
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
SYMTUZA is a fixed-dose combination of antiretroviral drugs darunavir (plus the CYP3A inhibitor cobicistat), emtricitabine, and tenofovir alafenamide [see Microbiology (12.4)] .
12.2 Pharmacodynamics
Cardiac Electrophysiology
Thorough QT trials have been conducted for darunavir, cobicistat, and tenofovir alafenamide. The effect of emtricitabine or the combination regimen SYMTUZA on the QT interval has not been evaluated.
Darunavir: In a thorough QT/QTc study in 40 healthy subjects, darunavir doses (co-administered with 100 mg ritonavir) of approximately 2 times the recommended darunavir dose did not affect the QT/QTc interval.
Cobicistat: In a thorough QT/QTc study in 48 healthy subjects, a single dose of cobicistat 250 mg and 400 mg (1.67 and 2.67 times the dose in SYMTUZA) did not affect the QT/QTc interval. Prolongation of the PR interval was noted in subjects receiving cobicistat. The maximum mean (95% upper confidence bound) difference in PR from placebo after baseline-correction was 9.5 (12.1) msec for the 250 mg cobicistat dose and 20.2 (22.8) for the 400 mg cobicistat dose. Because the 150 mg cobicistat dose used in the SYMTUZA fixed-dose combination tablet is lower than the lowest dose studied in the thorough QT study, it is unlikely that treatment with SYMTUZA will result in clinically relevant PR prolongation.
Effects on Serum Creatinine
The effect of cobicistat on serum creatinine was investigated in a trial in subjects with normal renal function (eGFR CG≥80 mL/min, N=12) and mild-to-moderate renal impairment (eGFR CG50–79 mL/min, N=18). A statistically significant decrease from baseline in the estimated glomerular filtration rate calculated by Cockcroft-Gault method (eGFR CG) was observed after 7 days of treatment with cobicistat 150 mg among subjects with normal renal function (-9.9 ± 13.1 mL/min) and mild-to-moderate renal impairment (-11.9 ± 7.0 mL/min). No statistically significant changes in eGFR CGwere observed compared to baseline for subjects with normal renal function or mild-to-moderate renal impairment 7 days after cobicistat was discontinued. The actual glomerular filtration rate, as determined by the clearance of probe drug iohexol, was not altered from baseline during treatment with cobicistat among subjects with normal renal function and mild-to-moderate renal impairment, indicating that cobicistat inhibits tubular secretion of creatinine, reflected as a reduction in eGFR CG, without affecting the actual glomerular filtration rate.
12.3 Pharmacokinetics
Absorption, Distribution, Metabolism, and Excretion
The bioavailability of the components of SYMTUZA was not affected when administered orally as a split tablet compared to administration as a tablet swallowed whole.
Pharmacokinetic (PK) properties and PK parameters of the components of SYMTUZA are provided in Table 5 and Table 6, respectively.
Table 5: Pharmacokinetic Properties of the Components of SYMTUZA Darunavir Cobicistat Emtricitabine TAF PBMCs = peripheral blood mononuclear cells; CES-1 = carboxylesterase-1 - *
- Approximately 928 kcal; 504 kcal from fat (56 g), 260 kcal from carbohydrates, and 164 kcal from protein.
- †
- Primarily alpha-1-acid glycoprotein
- ‡
- In vivo, TAF is hydrolyzed within cells to form tenofovir (major metabolite), which is phosphorylated to the active metabolite, tenofovir diphosphate. In vitro studies have shown that TAF is metabolized to tenofovir by cathepsin A in PBMCs and macrophages; and by CES1 in hepatocytes. Upon co-administration with the moderate CYP3A inducer probe efavirenz, TAF exposure was unaffected.
- §
- Note that the pharmacologically active metabolite tenofovir diphosphate has a half-life of 150–180 hours within PBMCs. Tenofovir in plasma has a median elimination half-life of approximately 44 hours.
- ¶
- Dosing in mass balance studies: darunavir (single dose administration of [ 14C] darunavir co-administered with multiple dose ritonavir 100 mg); cobicistat (single dose administration of [ 14C] cobicistat after multiple dosing of cobicistat for six days); emtricitabine (single dose administration of [ 14C] emtricitabine after multiple dosing of emtricitabine for ten days); TAF (single dose administration of [ 14C] TAF).
- #
- Unchanged darunavir accounted for approximately 41.2% and 7.7% of the administered dose in feces and urine, respectively.
Absorption T max(h) 3.0 3.0 1.5 0.5 Effect of high-fat meal *(compared to fasting) AUC lastLS mean ratio, 90% CI 1.52 (1.32–1.76) 1.41 (1.02–1.96) 1.00 (0.96–1.04) 1.12 (1.01–1.23) C maxLS mean ratio, 90% CI 1.82 (1.55–2.14) 1.30 (0.94–1.80) 0.79 (0.71–0.89) 0.55 (0.42–0.71) Distribution % bound to human plasma proteins 95 † 97–98 <4 ~80 Source of protein binding data In vitro In vitro In vitro Ex vivo Blood-to-plasma ratio 0.64 0.5 0.6 1.0 Metabolism Metabolism CYP3A CYP3A (major)
CYP2D6 (minor)Not significantly metabolized Cathepsin A ‡(PBMCs)
CES1 (hepatocytes)
CYP3A (minimal)Elimination t 1/2(h) 9.4 3.2 7.5 0.5 § Major route of elimination Metabolism Metabolism Glomerular filtration and active tubular secretion Metabolism (>80% of oral dose) % of dose excreted in feces ¶ 79.5 # 86.2 13.7 31.7 % of dose excreted in urine ¶ 13.9 # 8.2 70 <1 Table 6: Steady State Pharmacokinetic Parameters of Darunavir, Cobicistat, Emtricitabine, Tenofovir Alafenamide (TAF) and its Metabolite Tenofovir Following Oral Administration of SYMTUZA with Food in HIV-Infected Adults Parameter
Mean (SD)Darunavir Cobicistat * Emtricitabine * TAF Tenofovir * C max, ng/mL 8826 (33.3) * 1129 (35.3) 2056 (25.3) 163 (51.9) * 18.8 (37.6) AUC 24h, ng.h/mL 87909 (20232) † 85972 (22413) ‡ 8745 (43.9) 11918.0 (35.9) 132 (41) † 339 (37.1) C 0h, ng/mL 1899 (759) † 1813 (859) ‡ 31 (135) 93.1 (58.3) NA 11.7 (39.3) Specific Populations
Geriatric Patients
Darunavir: Pharmacokinetic analysis in HIV-infected subjects taking darunavir co-administered with cobicistat, emtricitabine, and tenofovir alafenamide showed no considerable differences in darunavir pharmacokinetics for ages below or equal to 65 years compared to ages greater than 65 years (N=25).
Pediatric Patients Weighing at Least 40 kg
Available pharmacokinetic data for the different components of SYMTUZA indicate that there were no clinically relevant differences in exposure between adults and pediatric subjects weighing at least 40 kg.
Darunavir and cobicistat: In pediatric subjects aged 12 to less than 18 years, weighing at least 40 kg who received darunavir 800 mg co-administered with cobicistat 150 mg (N=7), geometric mean darunavir C maxvalues were similar between adults and pediatric subjects. Geometric mean darunavir AUC 24hand C 24hvalues were 15% and 32% lower, with geometric mean ratios of 0.85 (90% CI: 0.64, 1.13) and 0.68 (90% CI: 0.30, 1.55) in pediatric subjects relative to adults, respectively. These differences were not considered clinically significant. Geometric mean cobicistat AUC 24h, C max, and C 24hvalues were comparable in pediatric subjects and adults (Table 7).
Table 7: Multiple-Dose PK Parameters of Darunavir and Cobicistat Following Administration of Darunavir with Cobicistat in HIV 1 Infected Adults and Pediatric Subjects Weighing at least 40 kg * Parameter Geometric mean (CV%) Darunavir Cobicistat CV = Coefficient of Variation; mcg = microgram - *
- From intensive PK analysis of trial GS-US-216-0128, where HIV-infected subjects were administered darunavir 800 mg and cobicistat 150 mg once daily with 2 NRTIs
- †
- N=5; Data from two subjects who had undetectable cobicistat C 24h concentrations were excluded from summary statistics
- ‡
- From intensive PK analysis of trial GS-US-299-0102 where HIV-infected subjects were administered SYMTUZA once daily
- §
- N=18
Pediatric Subjects * N=7 N=7 AUC 24h(mcg.hr/mL) 77.22 (29.5) 8.33 (34.9) C max(mcg/mL) 7.32 (21.7) 1.10 (20.0) C 24h(mcg/mL) 0.68 (91.6) 0.02 (123.9) † Adults ‡ N=21 N=21 AUC 24h(mcg.hr/mL) 90.56 (45.3) 7.69 (43.9) C max(mcg/mL) 8.34 (33.3) 1.04 (35.3) C 24h(mcg/mL) 1.00 (108.0) 0.02 (135.1) § Emtricitabine and tenofovir alafenamide: In 24 pediatric subjects aged 12 to less than 18 years, who received emtricitabine + TAF with elvitegravir + cobicistat, geometric mean emtricitabine C max, and C 24hvalues were comparable to adults, with geometric mean ratios of 1.10 (90% CI: 0.98, 1.23) and 1.07 (90% CI: 0.88, 1.29), respectively (Table 8). Geometric mean emtricitabine AUC 24hwas 21% higher, with a geometric mean ratio of 1.21 (90% CI: 1.09, 1.34) in pediatric subjects relative to adults. Geometric mean tenofovir alafenamide C maxand AUC lastvalues were 29% and 23% lower in pediatric subjects versus adults with geometric mean ratios of 0.71 (90% CI: 0.50, 1.00) and 0.77 (90% CI: 0.59, 1.02), respectively (Table 8). The observed differences were not considered clinically significant.
Table 8: Multiple-Dose PK Parameters of Emtricitabine and Tenofovir Alafenamide Following Oral Administration with Food in HIV 1 Infected Adults and Pediatric Subjects Parameter Geometric mean (CV%) Emtricitabine Tenofovir alafenamide CV = Coefficient of Variation; mcg = microgram; NA = not applicable - *
- From intensive PK analysis in trial GS-US-292-0106 in treatment-naïve pediatric subjects with HIV-1 infection
- †
- AUC last for tenofovir alafenamide
- ‡
- N=23
- §
- From intensive PK analysis in trial GS-US-292-0102 in HIV-infected adults treated with emtricitabine+tenofovir alafenamide and elvitegravir+cobicistat
Pediatric Subjects * N=24 N=24 AUC 24h(mcg.hr/mL) † 14.0 (23.9) 0.16 (55.8) C max(mcg/mL) 2.2 (22.5) 0.14 (64.4) C 24h(mcg/mL) 0.10 (38.9) ‡ NA Adults § N=19 N=19 AUC 24h(mcg.hr/mL) † 11.6 (16.6) 0.21 (47.3) C max(mcg/mL) 2.0 (20.2) 0.19 (64.6) C 24h(mcg/mL) 0.09 (46.7) NA Gender and Race
There were no clinically relevant differences in the pharmacokinetics of darunavir, cobicistat, emtricitabine, or tenofovir alafenamide based on gender or race.
Patients with Renal Impairment
Darunavir: The pharmacokinetics of darunavir were not altered in HIV-1 infected subjects with moderate renal impairment taking darunavir co-administered with ritonavir (creatinine clearance between 30–60 mL/min, estimated by Cockcroft-Gault method, N=20). There are no pharmacokinetic data available in HIV-1 infected patients with severe renal impairment or end-stage renal disease taking darunavir co-administered with cobicistat [see Use in Specific Populations (8.6)] .
Cobicistat: There were no clinically relevant differences in cobicistat pharmacokinetics observed between subjects with severe renal impairment (creatinine clearance below 30 mL/min, estimated by Cockcroft-Gault method) and healthy subjects [see Use in Specific Populations (8.6)] .
Emtricitabine: Mean systemic emtricitabine exposure was higher in patients with severe renal impairment (creatinine clearance less than 30 mL/min, estimated by Cockcroft-Gault method) than in subjects with normal renal function [see Use in Specific Populations (8.6)] .
Tenofovir alafenamide: In studies of TAF, no clinically relevant differences in the pharmacokinetics of TAF or its metabolite tenofovir were observed between subjects with severe renal impairment (creatinine clearance of 15–30 mL/min, estimated by Cockcroft-Gault method) and healthy subjects [see Use in Specific Populations (8.6)] .
Patients with Hepatic Impairment
Darunavir: There were no clinically relevant differences in the pharmacokinetics of darunavir (600 mg with ritonavir 100 mg twice daily) in subjects with mild hepatic impairment (Child-Pugh Class A, n=8), and moderate hepatic impairment (Child-Pugh Class B, n=8), compared to subjects with normal hepatic function (n=16). The effect of severe hepatic impairment on the pharmacokinetics of darunavir has not been evaluated [see Use in Specific Populations (8.7)] .
Cobicistat: There were no clinically relevant differences in the cobicistat pharmacokinetics between subjects with moderate hepatic impairment (Child-Pugh Class B) and healthy subjects. The effect of severe hepatic impairment on the pharmacokinetics of cobicistat has not been evaluated [see Use in Specific Populations (8.7)] .
Emtricitabine: The pharmacokinetics of emtricitabine have not been studied in subjects with hepatic impairment; however, emtricitabine is not significantly metabolized by liver enzymes, so the impact of hepatic impairment should be limited [see Use in Specific Populations (8.7)] .
Tenofovir Alafenamide: Clinically relevant changes in the pharmacokinetics of tenofovir alafenamide or its metabolite tenofovir were not observed in patients with mild, moderate (Child-Pugh Class A and B), or severe hepatic impairment (Child-Pugh Class C); [see Use in Specific Populations (8.7)] .
Patients with Hepatitis B and/or Hepatitis C Virus Coinfection
Darunavir: In HIV-infected subjects taking darunavir co-administered with ritonavir, the 48-week analysis of the data from clinical trials indicated that hepatitis B and/or hepatitis C virus coinfection status had no apparent effect on the exposure of darunavir.
Pregnancy and Postpartum
The exposure to total and unbound darunavir boosted with cobicistat after intake of darunavir/cobicistat as part of an antiretroviral regimen was substantially lower during the second and third trimesters of pregnancy compared with 6–12 weeks postpartum (see Table 9and Figure 1).
Table 9: Pharmacokinetic Results of Total Darunavir after Administration of Darunavir/Cobicistat Once Daily as Part of an Antiretroviral Regimen, During the 2 ndTrimester of Pregnancy, the 3 rdTrimester of Pregnancy, and Postpartum Pharmacokinetics of total darunavir
(mean ± SD)2 ndTrimester of pregnancy
N=73 rdTrimester of pregnancy
N=6Postpartum (6–12 weeks)
N=6C max, ng/mL 4340 ± 1616 4910 ± 970 7918 ± 2199 AUC 24h, ng.h/mL 47293 ± 19058 47991 ± 9879 99613 ± 34862 C min, ng/mL 168 ± 149 184 ± 99 1538 ± 1344 Figure 1: Pharmacokinetic Results (Within-Subject Comparison) of Total and Unbound Darunavir and Total Cobicistat After Administration of Darunavir/Cobicistat at 800/150 mg Once Daily as Part of an Antiretroviral Regimen, During the 2 ndand 3 rdTrimester of Pregnancy Compared to Postpartum
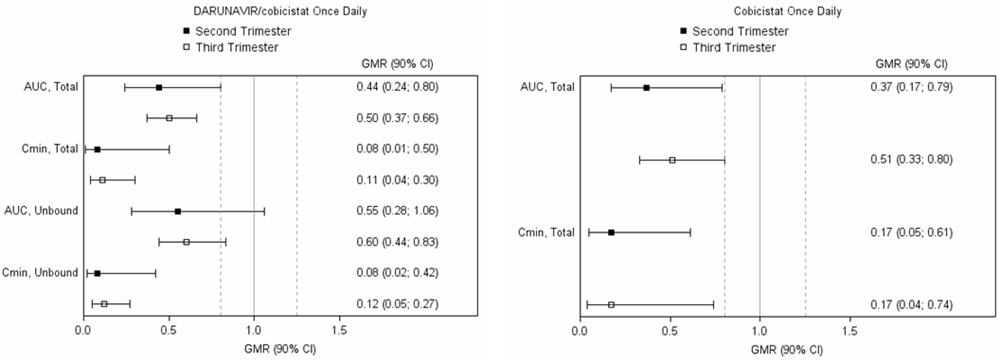
Legend: 90% CI: 90% confidence interval; GMR: geometric mean ratio (i.e., second or third trimester/postpartum). Solid vertical line: ratio of 1.0; dotted vertical lines: reference lines of 0.8 and 1.25.
Drug Interactions
Darunavir is metabolized by CYP3A. Cobicistat is metabolized by CYP3A and, to a minor extent, by CYP2D6. Darunavir co-administered with cobicistat is an inhibitor of CYP3A and CYP2D6. Cobicistat inhibits the following transporters: P-gp, BCRP, MATE1, OATP1B1, and OATP1B3. Based on in vitrodata, cobicistat is not expected to induce CYP1A2 or CYP2B6 and based on in vivodata, cobicistat is not expected to induce MDR1 or, in general, CYP3A to a clinically significant extent. The induction effect of cobicistat on CYP2C9, CYP2C19, or UGT1A1 is unknown, but is expected to be low based on CYP3A in vitroinduction data.
Emtricitabine is not an inhibitor of human CYP450 enzymes. In vitroand clinical drug interaction studies have shown that the potential for CYP-mediated interactions involving emtricitabine with other medicinal products is low. Tenofovir alafenamide is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or UGT1A. It is not an inhibitor or inducer of CYP3A in vivo.
A drug-drug interaction study between darunavir/cobicistat and dabigatran etexilate was conducted in healthy participants. The effects of darunavir on co-administration with dabigatran etexilate are summarized in Table 10.
Table 10: Drug Interactions: Pharmacokinetic Parameters for Co-Administered Drugs in the Presence of darunavir/cobicistat Dose/Schedule LS Mean ratio (90% CI) of co-administered drugpharmacokinetic parameters with/without darunavir
no effect =1.00Co-administered drug Co-administered drug Darunavir/ cobicistat N PK C max AUC C min N = number of subjects with data
q.d. = once daily- *
- 800/150 mg q.d. for 14 days before co-administered with dabigatran etexilate.
Dabigatran etexilate 150 mg 800/150 mg single dose 14 ↑ 2.64 (2.29–3.05) 2.64 (2.32–3.00) - 800/150 mg q.d. * 14 ↑ 1.99 (1.72–2.30) 1.88 (1.65–2.13) - 12.4 Microbiology
Mechanism of Action
Darunavir: Darunavir is an inhibitor of the HIV-1 protease. It selectively inhibits the cleavage of HIV-1 encoded Gag-Pol polyproteins in infected cells, thereby preventing the formation of mature virus particles.
Cobicistat: Cobicistat is a selective, mechanism-based inhibitor of CYPP450 of the CYP3A subfamily. Inhibition of CYP3A-mediated metabolism by cobicistat enhances the systemic exposure of CYP3A substrates.
Emtricitabine: Emtricitabine, a synthetic nucleoside analog of cytidine, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine 5'-triphosphate inhibits the activity of the HIV-1 reverse transcriptase (RT) by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA, which results in chain termination. Emtricitabine 5'-triphosphate is a weak inhibitor of mammalian DNA polymerases α, β, ε, and mitochondrial DNA polymerase γ.
Tenofovir alafenamide: TAF is a phosphonamidate prodrug of tenofovir (2'-deoxyadenosine monophosphate analog). Plasma exposure to TAF allows for permeation into cells and then TAF is intracellularly converted to tenofovir through hydrolysis by cathepsin A. Tenofovir is subsequently phosphorylated by cellular kinases to the active metabolite tenofovir diphosphate. Tenofovir diphosphate inhibits HIV-1 replication through incorporation into viral DNA by the HIV RT, which results in DNA chain-termination. Tenofovir diphosphate is a weak inhibitor of mammalian DNA polymerases that include mitochondrial DNA polymerase γ and there is no evidence of toxicity to mitochondria in cell culture.
Antiviral Activity
Darunavir: Darunavir exhibits activity against laboratory strains and clinical isolates of HIV-1 and laboratory strains of HIV-2 in acutely infected T-cell lines, human PBMCs, and human monocytes/macrophages with median EC 50values ranging from 1.2 to 8.5 nM (0.7 to 5.0 ng/mL). Darunavir demonstrates antiviral activity in cell culture against a broad panel of HIV-1 group M (A, B, C, D, E, F, G) and group O primary isolates with EC 50values ranging from less than 0.1 to 4.3 nM. The EC 50value of darunavir increases by a median factor of 5.4 in the presence of human serum.
Emtricitabine: The antiviral activity of emtricitabine against laboratory and clinical isolates of HIV-1 was assessed in T lymphoblastoid cell lines, the MAGI-CCR5 cell line, and primary PBMCs. The EC 50values for emtricitabine were in the range of 1.3–640 nM. Emtricitabine displayed antiviral activity in cell culture against HIV-1 clades A, B, C, D, E, F, and G (EC 50values ranged from 7–75 nM) and showed strain specific activity against HIV-2 (EC 50values ranged from 7–1,500 nM).
Tenofovir alafenamide: The antiviral activity of TAF against laboratory and clinical isolates of HIV-1 subtype B was assessed in lymphoblastoid cell lines, PBMCs, primary monocyte/macrophage cells, and CD4 +T lymphocytes. The EC 50values for TAF ranged from 2.0 to 14.7 nM. TAF displayed antiviral activity in cell culture against all HIV-1 groups (M, N, O), including sub-types A, B, C, D, E, F, and G (EC 50values ranged from 0.10 to 12.0 nM) and strain specific activity against HIV-2 (EC 50values ranged from 0.91 to 2.63 nM).
The combination of darunavir, emtricitabine, and tenofovir alafenamide was not antagonistic in cell culture combination antiviral activity assays. In addition, darunavir, emtricitabine, and tenofovir alafenamide were not antagonistic with a panel of representative agents from the major classes of approved HIV antivirals (PIs, NRTIs, NNRTIs, and INSTIs). The antiviral activity of approved HIV antivirals was not antagonized by cobicistat.
Resistance
Cell Culture
Darunavir: In cell culture, HIV-1 isolates with a decreased susceptibility to darunavir have been selected and obtained from subjects treated with darunavir co-administered with ritonavir. Darunavir-resistant virus derived in cell culture from wild-type HIV-1 had 21- to 88-fold decreased susceptibility to darunavir and developed 2 to 4 of the following amino acid substitutions S37D, R41E/T, K55Q, H69Q, K70E, T74S, V77I, or I85V in the protease. Selection in cell culture of darunavir-resistant HIV-1 from nine HIV-1 strains harboring multiple PI resistance-associated substitutions resulted in the overall emergence of 22 mutations in the protease gene, coding for amino acid substitutions L10F, V11I, I13V, I15V, G16E, L23I, V32I, L33F, S37N, M46I, I47V, I50V, F53L, L63P, A71V, G73S, L76V, V82I, I84V, T91A/S, and Q92R, of which L10F, V32I, L33F, S37N, M46I, I47V, I50V, L63P, A71V, and I84V were the most prevalent. These darunavir-resistant viruses had at least eight protease substitutions and exhibited 50- to 641-fold decreases in darunavir susceptibility with final EC 50values ranging from 125 nM to 3461 nM.
Clinical Trials
Darunavir resistance-associated substitutions (V11I, V32I, L33F, I47V, I50V, I54L or M, T74P, L76V, I84V, and L89V) in HIV-1 protease were derived from clinical trial data of antiretroviral therapy experienced patients, which were all protease inhibitor-experienced patients. Baseline International AIDS Society-USA (IAS-USA)-defined PI resistance substitutions confer reduced virologic response to darunavir.
In the AMBER clinical trial of subjects with no prior antiretroviral treatment history, there were 7 subjects with protocol-defined virologic failure and with HIV-1 RNA ≥400 copies/mL at failure or later timepoints who had post-baseline resistance data in the SYMTUZA arm. None of the subjects had detectable emergent darunavir resistance-associated substitutions or other primary protease inhibitor resistance-associated substitutions and only one subject had emergent M184M/I/V, which confers resistance to emtricitabine and lamivudine. In the comparative PREZCOBIX + emtricitabine/tenofovir disoproxil fumarate arm, there were 2 protocol-defined virologic failures with post-baseline resistance data and neither had detectable resistance emergence.
In the EMERALD clinical trial of virologically-suppressed subjects who switched to SYMTUZA, 1 subject who rebounded and 2 subjects who discontinued early from the study had post-baseline resistance genotypes. None of the subjects had darunavir, primary protease inhibitor, emtricitabine, or tenofovir resistance-associated substitutions. In the control arm, there were 3 subjects who rebounded with post-baseline genotypes and no resistance-associated substitutions were observed.
Cross-Resistance
Darunavir: Cross-resistance among PIs has been observed. Darunavir has a less than 10-fold decreased susceptibility in cell culture against 90% of 3309 clinical isolates resistant to amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, and/or tipranavir showing that viruses resistant to these PIs remain susceptible to darunavir. A less than 10-fold decreased susceptibility was observed for the other PIs in 26% to 96% of these PI resistant clinical isolates [nelfinavir (26%), ritonavir (34%), lopinavir (46%), indinavir (57%), atazanavir (59%), saquinavir (64%), amprenavir (70%), and tipranavir (96%)].
Cross-resistance between darunavir and nucleoside/nucleotide reverse transcriptase inhibitors, non-nucleoside reverse transcriptase inhibitors, gp41 fusion inhibitors, CCR5 co-receptor antagonists, or integrase strand transfer inhibitors is unlikely because the viral targets are different.
Emtricitabine: Emtricitabine-resistant viruses with the M184V or I substitution were cross-resistant to lamivudine, but retained sensitivity to didanosine, stavudine, tenofovir, and zidovudine.
Tenofovir Alafenamide: Tenofovir resistance-associated substitutions K65R and K70E result in reduced susceptibility to abacavir, didanosine, emtricitabine, lamivudine, and tenofovir. HIV-1 with multiple thymidine analog substitutions (M41L, D67N, K70R, L210W, T215F/Y, K219Q/E/N/R) or multinucleoside resistant HIV-1 with a T69S double insertion mutation or with a Q151M substitution complex including K65R, showed reduced susceptibility to TAF in cell culture.
-
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Darunavir: Darunavir was evaluated for carcinogenic potential by oral gavage administration to mice and rats up to 104 weeks. Daily doses of 150, 450, and 1000 mg/kg were administered to mice and doses of 50, 150, and 500 mg/kg were administered to rats. A dose-related increase in the incidence of hepatocellular adenomas and carcinomas was observed in males and females of both species and an increase in thyroid follicular cell adenomas was observed in male rats. The observed hepatocellular findings in rodents are considered to be of limited relevance to humans. Repeated administration of darunavir to rats caused hepatic microsomal enzyme induction and increased thyroid hormone elimination, which predispose rats but not humans to thyroid neoplasms. At the highest tested doses, the systemic exposures to darunavir (based on AUC) were between 0.5- and 0.6-fold (mice) and was 0.9-fold (rats) of exposures observed in humans at the recommended therapeutic dose of darunavir in SYMTUZA. Darunavir was not mutagenic or genotoxic in a battery of in vitroand in vivoassays including bacterial reverse mutation (Ames), chromosomal aberration in human lymphocytes, and in vivomicronucleus test in mice.
Cobicistat: In a long-term carcinogenicity study in mice, no drug-related increases in tumor incidence were observed at doses up to 50 and 100 mg/kg/day in males and females, respectively. Cobicistat exposures at these doses were approximately 8.6 (male) and 20 (females) times, respectively, the human systemic exposure at the therapeutic daily dose of cobicistat in SYMTUZA. In a long-term carcinogenicity study of cobicistat in rats, an increased incidence of follicular cell adenomas and/or carcinomas in the thyroid gland was observed at doses of 25 and 50 mg/kg/day in males, and at 30 mg/kg/day in females. The follicular cell findings are considered to be rat-specific, secondary to hepatic microsomal enzyme induction and thyroid hormone imbalance, and are not relevant for humans. At the highest doses tested in the rat carcinogenicity study, systemic exposures were approximately 2 times the human systemic exposure at the therapeutic daily dose of cobicistat in SYMTUZA. Cobicistat was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma, or rat micronucleus assays.
Emtricitabine: In long-term carcinogenicity studies of emtricitabine, no drug-related increases in tumor incidence were found in mice at doses up to 750 mg per kg per day (26 times the human systemic exposure at the recommended dose of emtricitabine in SYMTUZA) or in rats at doses up to 600 mg per kg per day (31 times the human systemic exposure at the recommended dose). Emtricitabine was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma, or mouse micronucleus assays. Emtricitabine did not affect fertility in male rats at approximately 107 times or in male and female mice at approximately 88 times higher exposures (AUC) than in humans given the recommended 200 mg daily dose in SYMTUZA. Fertility was normal in the offspring of mice exposed daily from before birth ( in utero) through sexual maturity at daily exposures (AUC) of approximately 88 times higher than human exposures at the recommended 200 mg daily dose.
Tenofovir alafenamide: Since TAF is rapidly converted to tenofovir and a lower tenofovir exposure in rats and mice was observed after TAF administration compared to TDF administration, carcinogenicity studies were conducted only with TDF. Long-term oral carcinogenicity studies of TDF in mice and rats were carried out at exposures up to approximately 10 times (mice) and 4 times (rats) those observed in humans at the 300 mg therapeutic dose of TDF for HIV-1 infection. The tenofovir exposure in these studies was approximately 167 times (mice) and 55 times (rat) those observed in humans after administration of the daily recommended dose of TAF. At the high dose in female mice, liver adenomas were increased at tenofovir exposures approximately 10 times (300 mg TDF) and 167 times (10 mg TAF) the exposure observed in humans. In rats, the study was negative for carcinogenic findings.
TAF was not genotoxic in the reverse mutation bacterial test (Ames test), mouse lymphoma, or rat micronucleus assays. There were no effects on fertility, mating performance, or early embryonic development when TAF was administered to male rats at a dose equivalent to 155 times the human dose based on body surface area comparisons for 28 days prior to mating and to female rats for 14 days prior to mating through Day 7 of gestation.
13.2 Animal Toxicology and/or Pharmacology
Minimal to slight infiltration of mononuclear cells in the posterior uvea was observed in dogs with similar severity after 3 and 9 month administration of tenofovir alafenamide; reversibility was seen after a 3-month recovery period. No eye toxicity was observed in the dog at systemic exposures of 3.5 (TAF) and 0.62 (tenofovir) times the exposure seen in humans with the recommended daily dose of TAF in SYMTUZA.
-
14. CLINICAL STUDIES
14.1 Clinical Trial Results in Subjects with HIV-1 Infection with no Prior Antiretroviral Treatment History
The efficacy of SYMTUZA in HIV-1 subjects with no prior antiretroviral treatment history was evaluated in the Phase 3 trial TMC114FD2HTX3001 [NCT02431247, (AMBER)] in which subjects were randomized in a 1:1 ratio to receive either SYMTUZA (N=362) or a combination of PREZCOBIX and FTC/TDF (N=363) once daily. The median age was 34.0 years (range 18–71), 88.3% were male, 83% White, 11% Black, and 2% Asian. The mean baseline plasma HIV-1 RNA was 4.5 log 10copies/mL (range 1.3–6.7), and 18% had a baseline viral load ≥100,000 copies/mL. The median baseline CD4+ cell count was 453 cells/mm 3(range 38 to 1456 cells/mm 3).
Virologic outcomes at 48 weeks of treatment are presented in Table 11.
Table 11: Virologic Outcomes in AMBER at Week 48 in HIV-1 Subjects with No Prior Antiretroviral Treatment History SYMTUZA
N=362PREZCOBIX + FTC/TDF
N=363Virologic Response - *
- Based on stratum adjusted MH test where stratification factors are HIV-1 RNA level (≤100,000 or > 100,000 copies/mL) and CD4+ cell count (< 200 or ≥200 cells/µL).
- †
- Included subjects who had ≥50 copies/mL in the Week 48 window; subjects who discontinued early due to lack or loss of efficacy; subjects who discontinued for reasons other than an adverse event (AE), death, or lack or loss of efficacy and at the time of discontinuation had a viral value of ≥ 50 copies/mL.
- ‡
- Day 295 – Day 378
- §
- Other includes reasons such as withdrew consent, loss to follow-up, and non-compliance.
HIV-1 RNA <50 copies/mL 91% 88% Treatment difference * 2.7 (95% CI: -1.6; 7.1) Virologic Failure † 4% 3% No virologic data at Week 48 window ‡ 4% 8% Reasons Discontinued trial due to adverse event or death 2% 4% Discontinued trial for other reasons § 1% 3% Missing data during window but on trial 1% 1% The mean increase from baseline in CD4+ cell count at Week 48 was 189 and 174 cells/mm 3in the SYMTUZA and PREZCOBIX + FTC/TDF groups, respectively.
14.2 Clinical Trial Results in Virologically-Suppressed Subjects with HIV-1 Infection Who Switched to SYMTUZA
Phase 3 trial TMC114IFD3013 [NCT02269917, (EMERALD)] evaluated the efficacy of SYMTUZA in virologically-suppressed (HIV-1 RNA less than 50 copies/mL) subjects with HIV-1 infection. Subjects were virologically suppressed for at least 2 months and no more than once had a viral load elevation above 50 HIV-1 RNA copies/mL during the year prior to enrollment. Subjects were on a stable antiretroviral regimen (for at least 6 months), consisting of a bPI [either darunavir once daily or atazanavir (both boosted with ritonavir or cobicistat), or lopinavir with ritonavir] combined with emtricitabine and TDF. Subjects had no history of failure on darunavir treatment and no known or suspected darunavir resistance-associated substitutions. Emtricitabine or tenofovir resistance-associated substitutions were not specifically excluded by the protocol. They either switched to SYMTUZA (N=763) or continued their treatment regimen (N=378) (randomized 2:1). Subjects had a median age of 46 years (range 19–78), 82% were male, 75% White, 21% Black, and 2% Asian. The median baseline CD4+ cell count was 628 cells/mm 3(range 111–1921 cells/mm 3). Overall, 15% (N=169) of subjects had prior virologic failure. Five subjects had archived tenofovir resistance-associated substitutions and 53 subjects had archived emtricitabine resistance-associated substitutions, mainly at RT position M184. All of these subjects with emtricitabine resistance-associated substitutions had HIV-1 RNA<50 copies/mL at Week 48 (N=50) or at the last on-treatment viral load (N=3). Virologic outcomes are presented in Table 12. Prior virologic failure did not impact treatment outcomes.
Table 12: Virologic Outcomes in EMERALD at Week 48 in HIV-1 Virologically-Suppressed Subjects Who Switched to SYMTUZA SYMTUZA
N=763bPI+FTC/TDF
N=378- *
- Included subjects who had ≥50 copies/mL in the Week 48 window; subjects who discontinued early due to lack or loss of efficacy; subjects who discontinued for reasons other than an adverse event (AE), death, or lack or loss of efficacy and at the time of discontinuation had a viral value ≥ 50 copies/mL.
- †
- Based on MH test adjusting for bPI at screening (ATV with rtv or COBI, DRV with rtv or COBI, LPV with rtv).
- ‡
- Day 295 – Day 378
- §
- Other includes reasons such as withdrew consent, loss to follow-up, and non-compliance
Virologic Failure* 1% 1% Treatment difference † 0.3 (95% CI: -0.7; 1.2) HIV-1 RNA <50 copies/mL 95% 94% No virologic data at Week 48 window ‡ 4% 6% Reasons Discontinued trial due to adverse event or death 1% 1% Discontinued trial for other reasons § 3% 4% Missing data during window ‡but on trial <1% 1% The mean increase from baseline in CD4+ cell count at Week 48 was 20 cells/mm 3in subjects who switched to SYMTUZA and 8 cells/mm 3in subjects who stayed on their baseline PI + FTC/TDF.
14.3 Clinical Trial Results in Pediatric Subjects with HIV-1 Infection
The pharmacokinetic profile, safety, and antiviral activity of the components of SYMTUZA were evaluated in open-label clinical trials in pediatric subjects with HIV-1 infection aged 12 to less than 18 years: GS-US-216-0128 (N=7) and GS-US-292-0106 (N=50).
In the Phase 2/3 trial GS-US-216-0128, darunavir 800 mg and cobicistat 150 mg once daily with 2 NRTIs were evaluated in 7 virologically suppressed pediatric subjects aged 12 to less than 18 years and weighing at least 40 kg. Subjects had a median (range) age of 14 (12–16) years and a median (range) weight of 57 (45–78) kg. At baseline, plasma HIV-1 RNA was <50 copies/mL in all subjects, and the median (range) CD4+ cell count was 1,117 (658–2,416) cells/mm 3. At Week 48, the proportion of subjects who maintained HIV-1 RNA <50 copies/mL was 86%, and the median change in CD4+ cell count from baseline was -342 cells/mm 3(range -1,389 to 210 cells/mm 3). All 6 subjects with available data had CD4+ cell counts above 800 cells/mm 3at Week 48.
In the Phase 2/3 trial GS-US-292-0106, cobicistat 150 mg, emtricitabine 200 mg, and tenofovir alafenamide 10 mg, as part of a fixed-dose combination regimen together with elvitegravir 150 mg, were evaluated in 50 treatment-naïve pediatric subjects with HIV-1 aged 12 to less than 18 years and weighing at least 35 kg. Subjects had a median (range) age of 15 (12–17) years. At baseline, median (range) plasma HIV-1 RNA was 4.7 (3.3–6.5) log 10copies/mL, median (range) CD4+ cell count was 456 (95–1,110) cells/mm 3, and 22% had baseline plasma HIV-1 RNA >100,000 copies/mL). At Week 48, the proportion of subjects who had HIV-1 RNA <50 copies/mL was 92%, and the median increase in CD4+ cell count from baseline was 220 cells/mm 3.
The use of SYMTUZA in pediatric patients weighing less than 40 kg has not been established [see Use in Specific Populations (8.4)].
-
16. HOW SUPPLIED/STORAGE AND HANDLING
SYMTUZA ®(darunavir, cobicistat, emtricitabine, and tenofovir alafenamide) tablets are supplied as yellow to yellowish-brown, capsule-shaped, film-coated tablets debossed with "8121" on one side and "JG" on the other side.
SYMTUZA is packaged in bottles of 30 tablets (NDC 59676-800-30), with a silica gel desiccant and child-resistant closure.
-
17. PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Instructions for Use
Advise patients to take SYMTUZA with food every day on a regular dosing schedule, as missed doses can result in development of resistance. Inform patients not to alter the dose of SYMTUZA or discontinue therapy with SYMTUZA without consulting their physician. For patients who are unable to swallow tablets whole, SYMTUZA may be split using a tablet-cutter, and the entire dose should be consumed immediately after splitting [see Dosage and Administration (2.2)] .
Post-treatment Acute Exacerbation of Hepatitis B in Patients with HBV Co-Infection
Severe acute exacerbations of hepatitis B have been reported in patients who are coinfected with HBV and HIV-1 and have discontinued products containing emtricitabine and/or TDF, and may likewise occur with discontinuation of SYMTUZA [see Warnings and Precautions (5.1)] . Advise the patient to not discontinue SYMTUZA without first informing their healthcare provider.
Hepatotoxicity
Inform patients that drug-induced hepatitis (e.g., acute hepatitis, cytolytic hepatitis) and liver injury, including some fatalities, could potentially occur with SYMTUZA. Advise patients to contact their healthcare provider immediately if signs and symptoms of liver problems develop [see Warnings and Precautions (5.2)] .
Severe Skin Reactions
Inform patients that skin reactions ranging from mild to severe, including Stevens-Johnson syndrome, drug rash with eosinophilia and systemic symptoms, and toxic epidermal necrolysis, could potentially occur with SYMTUZA. Advise patients to contact their healthcare provider immediately if signs or symptoms of severe skin reactions develop, including but not limited to severe rash or rash accompanied with fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, and/or conjunctivitis [see Warnings and Precautions (5.3)] .
Pregnancy
Advise patients that SYMTUZA is not recommended during pregnancy and to alert their healthcare provider if they get pregnant while taking SYMTUZA. Inform patients that there is an antiretroviral pregnancy registry to monitor fetal outcomes of pregnant individuals exposed to SYMTUZA [see Use in Specific Populations (8.1)] .
Lactation
Instruct individuals with HIV-1 infection not to breastfeed because HIV-1 can be passed to the baby in breast milk [see Use in Specific Populations (8.2)] .
Drug Interactions
SYMTUZA may interact with many drugs; therefore, inform patients of the potential serious drug interactions with SYMTUZA, and that some drugs are contraindicated with SYMTUZA and other drugs may require dosage adjustment. Advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products, including St. John's wort [see Contraindications (4), Warnings and Precautions (5.4), and Drug Interactions (7)] .
Immune Reconstitution Syndrome
Advise patients to inform their healthcare provider immediately of any symptoms of infection, as in some patients with advanced HIV infection (AIDS), signs and symptoms of inflammation from previous infections may occur soon after anti-HIV treatment is started [see Warnings and Precautions (5.5)] .
Renal Impairment
Advise patients to avoid taking SYMTUZA with concurrent or recent use of nephrotoxic agents. Renal impairment, including cases of acute renal failure, has been reported in association with the use of tenofovir prodrugs [see Warnings and Precautions (5.6)] .
Lactic Acidosis and Severe Hepatomegaly
Lactic acidosis and severe hepatomegaly with steatosis, including fatal cases, have been reported with use of drugs similar to SYMTUZA. Advise patients that they should stop SYMTUZA if they develop clinical symptoms suggestive of lactic acidosis or pronounced hepatotoxicity [see Warnings and Precautions (5.8)] .
Fat Redistribution
Inform patients that redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy, including SYMTUZA, and that the cause and long-term health effects of these conditions are not known at this time [see Warnings and Precautions (5.10)] .
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 03/2023 PATIENT INFORMATION
SYMTUZA ®(sim toó zah)
(darunavir, cobicistat, emtricitabine, and tenofovir alafenamide)
tabletsWhat is the most important information I should know about SYMTUZA?
SYMTUZA can cause serious side effects, including:-
Worsening of Hepatitis B virus infection (HBV).Your healthcare provider will test you for HBV before starting treatment with SYMTUZA. If you have HBV infection and take SYMTUZA, your HBV may get worse (flare-up) if you stop taking SYMTUZA. A "flare-up" is when your HBV infection suddenly returns in a worse way than before.
- Do not stop taking SYMTUZA without first talking to your healthcare provider.
- Do not run out of SYMTUZA. Refill your prescription or talk to your healthcare provider before your SYMTUZA is all gone.
- If you stop taking SYMTUZA, your healthcare provider will need to check your health often and do blood tests regularly for several months to check your HBV infection, or give you a medicine to treat your HBV infection. Tell your healthcare provider about any new or unusual symptoms you may have after you stop taking SYMTUZA.
- Change in liver enzymes. People with a history of hepatitis B or C virus infection or who have certain liver enzyme changes may have an increased risk of developing new or worsening liver problems during treatment with SYMTUZA. Liver problems can also happen during treatment with SYMTUZA in people without a history of liver disease. Your healthcare provider may need to do tests to check your liver enzymes before and during treatment with SYMTUZA.
- Severe liver problems.In rare cases, severe liver problems can happen that can lead to death. Tell your healthcare provider right away if you get these symptoms:skin or the white part of your eyes turns yellow, dark "tea-colored" urine, light-colored stools, loss of appetite for several days or longer, nausea, vomiting, or stomach-area pain.
- fever
- tiredness
- muscle or joint pain
- blisters or skin lesions
- mouth sores or ulcers
- red or inflamed eyes, like "pink eye" (conjunctivitis)
See " What are the possible side effects of SYMTUZA?" for more information about side effects. What is SYMTUZA?
SYMTUZA is a prescription medicine that is used without other antiretroviral medicines to treat Human Immunodeficiency Virus-1 (HIV-1) infection in adults and in children who weigh at least 88 pounds (40 kg) who:- have not received anti-HIV-1 medicines in the past, or
- when their healthcare provider determines that they meet certain requirements.
SYMTUZA contains the prescription medicines darunavir, cobicistat, emtricitabine, and tenofovir alafenamide.
It is not known if SYMTUZA is safe and effective in children weighing less than 88 pounds (40 kg).Who should not take SYMTUZA?
Do not take SYMTUZA with any of the following medicines:- alfuzosin
- carbamazepine
- colchicine, if you have liver or kidney problems
- dronedarone
- elbasvir and grazoprevir
- ergot-containing medicines, such as:
- dihydroergotamine
- ergotamine tartrate
- methylergonovine
- ivabradine
- lomitapide
- lovastatin or a product that contains lovastatin
- lurasidone
- midazolam, when taken by mouth
- naloxegol
- phenobarbital
- phenytoin
- pimozide
- ranolazine
- rifampin
- sildenafil, when used for the treatment of pulmonary arterial hypertension (PAH)
- simvastatin or a product that contains simvastatin
- St. John's wort ( Hypericum perforatum), or a product that contains St. John's wort
- triazolam
Before taking SYMTUZA, tell your healthcare provider about all of your medical conditions, including if you: - have liver problems, including hepatitis B or hepatitis C
- have kidney problems
- are allergic to sulfa (sulfonamide)
- have diabetes
- have hemophilia
- are pregnant or plan to become pregnant.
- It is not known if SYMTUZA will harm your unborn baby.
- SYMTUZA should not be used during pregnancy because you may not have enough SYMTUZA in your body during pregnancy.
- Tell your healthcare provider if you become pregnant while taking SYMTUZA. Your healthcare provider will prescribe different medicines if you become pregnant while taking SYMTUZA.
Pregnancy Registry: There is a pregnancy registry for those who take antiretroviral medicines during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. Talk to your healthcare provider about how you can take part in this registry.
- are breastfeeding or plan to breastfeed. Do not breastfeed if you take SYMTUZA.
- You should not breastfeed if you have HIV-1 because of the risk of passing HIV to your baby.
- One of the medicines in SYMTUZA called emtricitabine can pass into your breast milk. It is not known if the other medicines in SYMTUZA can pass into your breast milk.
- Talk to your healthcare provider about the best way to feed your baby.
- You can ask your healthcare provider or pharmacist for a list of medicines that interact with SYMTUZA.
- Do not start taking a new medicine without telling your healthcare provider.Your healthcare provider can tell you if it is safe to take SYMTUZA with other medicines.
How should I take SYMTUZA? - Take SYMTUZA exactly as your healthcare provider tells you.
- Do not change your dose or stop taking SYMTUZA without talking to your healthcare provider.
- Take SYMTUZA 1 time a day with food.
- If you have difficulty swallowing, the tablet may be split using a tablet-cutter. After splitting the tablet, the entire dose (both halves) should then be taken right away.
- Do not miss a dose of SYMTUZA.
- When your SYMTUZA supply starts to run low, get more from your healthcare provider or pharmacy. This is very important because the amount of virus in your blood may increase if the medicine is stopped for even a short time. The virus may develop resistance to SYMTUZA and become harder to treat.
- If you take too much SYMTUZA, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of SYMTUZA?
SYMTUZA may cause serious side effects, including:- See " What is the most important information I should know about SYMTUZA?"
- Changes in your immune system (Immune Reconstitution Syndrome)can happen when you start taking HIV-1 medicines. Your immune system may get stronger and begin to fight infections that have been hidden in your body for a long time. Tell your healthcare provider right away if you start having new symptoms after starting your HIV-1 medicine.
- New or worse kidney problems, including kidney failure.Your healthcare provider should do blood and urine tests to check your kidneys before you start and while you are taking SYMTUZA. Your healthcare provider may tell you to stop taking SYMTUZA if you develop new or worse kidney problems.
- Too much lactic acid in your blood (lactic acidosis).Too much lactic acid is a serious but rare medical emergency that can lead to death. Tell your healthcare provider right away if you get these symptoms:weakness or being more tired than usual, unusual muscle pain, being short of breath or fast breathing, stomach pain with nausea and vomiting, cold or blue hands and feet, feel dizzy or lightheaded, or a fast or abnormal heartbeat.
- Diabetes and high blood sugar (hyperglycemia).Some people who take protease inhibitors including SYMTUZA can get high blood sugar, develop diabetes, or your diabetes can get worse. Tell your healthcare provider if you notice an increase in thirst or if you start urinating more often while taking SYMTUZA.
- Changes in body fatcan happen in people who take HIV-1 medicines. The changes may include an increased amount of fat in the upper back and neck ("buffalo hump"), breast, and around the middle of your body (trunk). Loss of fat from the legs, arms, and face may also happen. The exact cause and long-term health effects of these conditions are not known.
- Increased bleeding for hemophiliacs.Some people with hemophilia have increased bleeding with protease inhibitors.
- diarrhea
- rash
- nausea
- fatigue
- headache
- stomach problems
- gas
These are not all of the possible side effects of SYMTUZA.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store SYMTUZA? - Store SYMTUZA tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- The SYMTUZA bottle contains a desiccant and has a child-resistant cap.
- Keep the SYMTUZA container tightly closed with the desiccant inside of it to protect SYMTUZA from moisture.
General information about the safe and effective use of SYMTUZA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use SYMTUZA for a condition for which it was not prescribed. Do not give SYMTUZA to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about SYMTUZA that is written for health professionals.What are the ingredients in SYMTUZA?
Active ingredient : darunavir, cobicistat, emtricitabine, and tenofovir alafenamide
Inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, and microcrystalline cellulose. The tablets are film-coated with a coating material containing polyethylene glycol (macrogol), polyvinyl alcohol (partially hydrolyzed), talc, titanium dioxide, and yellow ferric oxide.
Manufactured for: Janssen Products, LP, Horsham PA 19044, USA
© 2018, 2020 Janssen Pharmaceutical Companies
For more information call 1-800-526-7736. -
Worsening of Hepatitis B virus infection (HBV).Your healthcare provider will test you for HBV before starting treatment with SYMTUZA. If you have HBV infection and take SYMTUZA, your HBV may get worse (flare-up) if you stop taking SYMTUZA. A "flare-up" is when your HBV infection suddenly returns in a worse way than before.
-
PRINCIPAL DISPLAY PANEL - 30 Tablet Bottle Label
NDC 59676-800-30
Symtuza™
(darunavir, cobicistat,
emtricitabine, and tenofovir
alafenamide) tablets800 mg / 150 mg /
200 mg / 10 mgEach tablet contains darunavir
ethanolate equivalent to 800 mg
of darunavir, 150 mg of cobicistat,
200 mg of emtricitabine, and
tenofovir alafenamide fumarate
equivalent to 10 mg of tenofovir
alafenamide.Rx only
30 Tablets
-
INGREDIENTS AND APPEARANCE
SYMTUZA
darunavir, cobicistat, emtricitabine, and tenofovir alafenamide tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59676-800 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DARUNAVIR (UNII: YO603Y8113) (DARUNAVIR - UNII:YO603Y8113) DARUNAVIR 800 mg COBICISTAT (UNII: LW2E03M5PG) (COBICISTAT - UNII:LW2E03M5PG) COBICISTAT 150 mg EMTRICITABINE (UNII: G70B4ETF4S) (EMTRICITABINE - UNII:G70B4ETF4S) EMTRICITABINE 200 mg TENOFOVIR ALAFENAMIDE (UNII: EL9943AG5J) (TENOFOVIR ANHYDROUS - UNII:W4HFE001U5) TENOFOVIR ALAFENAMIDE 10 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color brown (yellow to yellowish-brown) Score no score Shape OVAL (capsule-shaped, film-coated tablet) Size 22mm Flavor Imprint Code 8121;JG Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59676-800-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/17/2018 2 NDC:59676-800-99 30 in 1 BOTTLE; Type 0: Not a Combination Product 07/17/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210455 07/17/2018 Labeler - Janssen Products LP (804684207) Establishment Name Address ID/FEI Business Operations AMPAC Fine Chemicals 073903937 api manufacture(59676-800) Establishment Name Address ID/FEI Business Operations Gilead Alberta ULC 207452996 api manufacture(59676-800) Establishment Name Address ID/FEI Business Operations Patheon, Inc. 240769596 manufacture(59676-800) , analysis(59676-800) Establishment Name Address ID/FEI Business Operations Evonik Operations GmbH 343170605 api manufacture(59676-800) Establishment Name Address ID/FEI Business Operations Janssen Pharmaceutica NV 400345889 manufacture(59676-800) Establishment Name Address ID/FEI Business Operations Union Quimico Farmaceutica SA (Uquifa) 460940868 api manufacture(59676-800) Establishment Name Address ID/FEI Business Operations Janssen Cilag SpA 542797928 pack(59676-800) , manufacture(59676-800) Establishment Name Address ID/FEI Business Operations Esteve Quimica, SA 633485529 api manufacture(59676-800) Establishment Name Address ID/FEI Business Operations Yuhan Chemical, Inc. 687831958 api manufacture(59676-800) Establishment Name Address ID/FEI Business Operations Janssen Ortho LLC 805887986 pack(59676-800) Establishment Name Address ID/FEI Business Operations Janssen Pharmaceuticals, Inc. 868441320 analysis(59676-800) Establishment Name Address ID/FEI Business Operations Janssen Pharmaceutical Sciences Unlimited Company 985639841 api manufacture(59676-800) , analysis(59676-800) Establishment Name Address ID/FEI Business Operations Porton Pharma Solutions Ltd 528192390 api manufacture(59676-800) Establishment Name Address ID/FEI Business Operations Thermo Fisher Scientific Cork Limited 985745028 api manufacture(59676-800)

