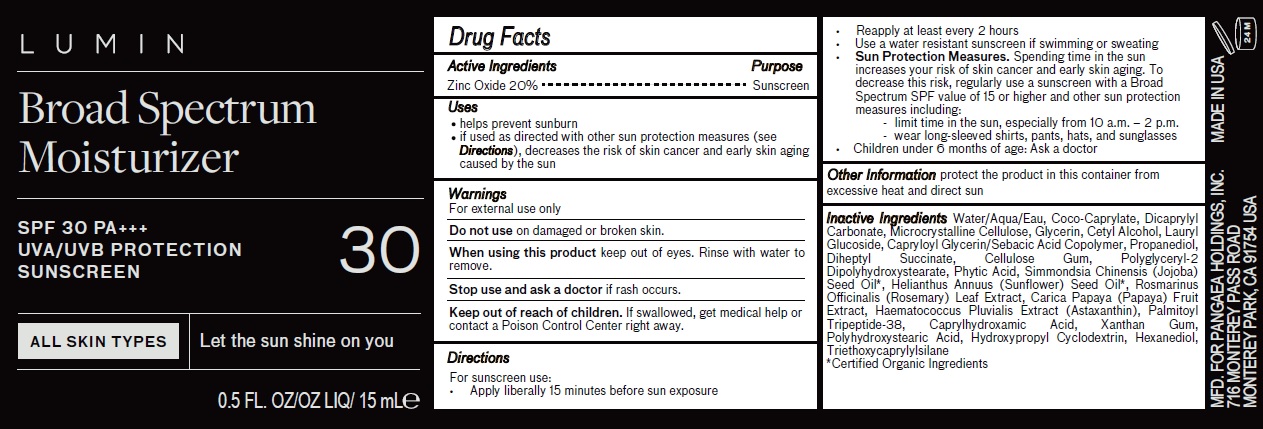
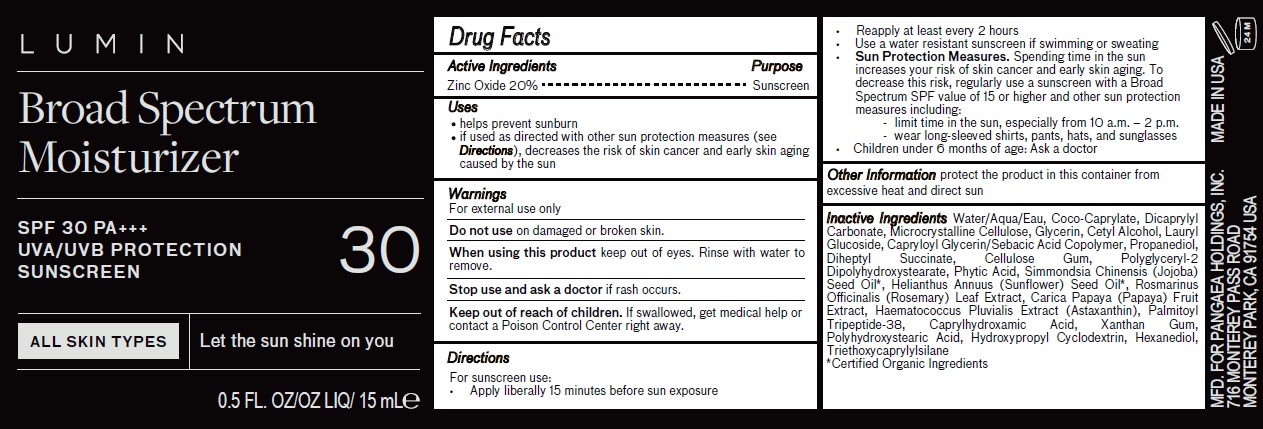
Label: LUMIN BROAD SPECTRUM MOISTURIZER WITH SPF 30 PA UVA/UVB PROTECTION SUNSCREEN- zinc oxide cream
- NDC Code(s): 81234-808-00, 81234-808-01, 81234-808-02
- Packager: Pangaea Holdings Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
-
Directions
For sunscreen use:
• Apply liberally 15 minutes before sun exposure• Reapply at least every 2 hours
• Use a water resistant sunscreen if swimming or sweating• Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
• Children under 6 months ofOther Information age: Ask a doctor - Other Information
-
Inactive Ingredients
Water/Aqua/Eau, Coco-Caprylate, Dicaprylyl Carbonate, Microcrystalline Cellulose, Glycerin, Cetyl Alcohol, Lauryl Glucoside, Capryloyl Glycerin/Sebacic Acid Copolymer, Propanediol, Diheptyl Succinate, Cellulose Gum, Polyglyceryl-2 Dipolyhydroxystearate, Phytic Acid, Simmondsia Chinensis (Jojoba) Seed Oil*, Helianthus Annuus (Sunflower) Seed Oil*, Rosmarinus Officinalis (Rosemary) Leaf Extract, Carica Papaya (Papaya) Fruit Extract, Haematococcus Pluvialis Extract (Astaxanthin), Palmitoyl Tripeptide-38, Caprylhydroxamic Acid, Xanthan Gum, Polyhydroxystearic Acid, Hydroxypropyl Cyclodextrin, Hexanediol, Triethoxycaprylylsilane
*Certified Organic Ingredients - Package Labeling:81234-808-00
- Package Labeling:81234-808-01
- Package Labeling:81234-808-02
-
INGREDIENTS AND APPEARANCE
LUMIN BROAD SPECTRUM MOISTURIZER WITH SPF 30 PA UVA/UVB PROTECTION SUNSCREEN
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81234-808 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength PALMITOYL LYSYLDIOXYMETHIONYLLYSINE (UNII: T7A529FB8O) CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) XANTHAN GUM (UNII: TTV12P4NEE) HEXANEDIOL (UNII: ZIA319275I) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) HYDROXYPROPYLBETADEX (0.58-0.68 MS) (UNII: 1I96OHX6EK) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) WATER (UNII: 059QF0KO0R) COCO-CAPRYLATE (UNII: 4828G836N6) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) PROPANEDIOL (UNII: 5965N8W85T) DIHEPTYL SUCCINATE (UNII: 057N7SS26Y) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) POLYGLYCERYL-2 DIPOLYHYDROXYSTEARATE (UNII: 9229XJ4V12) FYTIC ACID (UNII: 7IGF0S7R8I) JOJOBA OIL (UNII: 724GKU717M) SUNFLOWER OIL (UNII: 3W1JG795YI) ROSEMARY (UNII: IJ67X351P9) PAPAYA (UNII: KU94FIY6JB) HAEMATOCOCCUS PLUVIALIS (UNII: 31T0FF0472) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81234-808-00 1 in 1 BOX 01/01/2022 01/05/2025 1 15 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 2 NDC:81234-808-01 1 in 1 BOX 01/01/2022 01/05/2025 2 30 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 3 NDC:81234-808-02 1 in 1 BOX 01/01/2022 01/05/2025 3 40 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/01/2022 01/05/2025 Labeler - Pangaea Holdings Inc. (081181313)