Label: CHLORAPREP ONE-STEP- chlorhexidine gluconate and isopropyl alcohol solution
- NDC Code(s): 54365-400-37, 54365-400-38, 54365-400-39
- Packager: CareFusion 213, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 20, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
WARNING
FLAMMABLE
Keep away from fire or flame.
To reduce the risk of fire, PREP CAREFULLY:
- do not use 26-mL applicator for head and neck surgery or on an area
smaller than 8.4 in. x 8.4 in. Use a smaller applicator instead.
- solution contains alcohol and gives off flammable vapors
- avoid getting solution into hairy areas. Hair may take up to 1 hour to
dry. Wet hair is flammable.
- do not drape or use ignition source (e.g., cautery, laser) until solution
is completely dry (minimum of 3 minutes on hairless skin; up to 1
hour in hair)
- do not allow solution to pool
- remove wet materials from prep area
- Active ingredients
- Purposes
- Use
-
Warnings
For external use only. Flammable, keep away from fire or flame.
To reduce risk of fire, PREP CAREFULLY:
- do not use 26-ml applicator for head and neck surgery
- do not use on an area smaller than 8.4 in. x 8.4 in. Use a smaller applicator instead.
- solution contains alcohol and gives off flammable vapors
- avoid getting solution into hairy areas. Hair may take up to 1 hour to dry.
Wet hair is flammable.
- do not drape or use ignition source (e.g., cautery, laser) until solution is
completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair)
- do not allow solution to pool
- remove wet materials from prep area
- Allergy alert:
- Do Not Use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children.
-
Directions
• use with care in premature infants or infants under 2 months of age. These
products may cause irritation or chemical burns • use in a well ventilated
area • maximal treatment area for one applicator is approximately
13.2 in. x 13.2 in. (1126 cm2). Do not use 26-ml applicator for area smaller than
8.4 in. x 8.4 in. Use a smaller applicator instead. • do not use 26-ml applicator
for head and neck surgery • remove applicator from package; do not touch
sponge • hold the applicator with the sponge down. Pinch wing only once to
activate the ampules and release the antiseptic • wet the sponge by pressing
and releasing the sponge against the treatment area until liquid is visible on the
skin • completely wet the treatment area with antiseptic • dry surgical
sites (e.g., abdomen or arm): use gentle repeated back-and-forth strokes for
30 seconds • moist surgical sites (e.g., inguinal fold): use gentle repeated
back-and-forth strokes for 2 minutes • do not allow solution to pool; tuck
prep towels to absorb solution, and then remove • allow the solution to
completely dry (minimum of 3 minutes on hairless skin; up to 1 hour in hair).
Do not blot or wipe away • discard the applicator after a single use along with
any portion of the solution not required to cover the prep area. It is not necessary
to use the entire amount available.
- Other Information
- Inactive Ingredients
- Questions?
-
26 mL Package Label
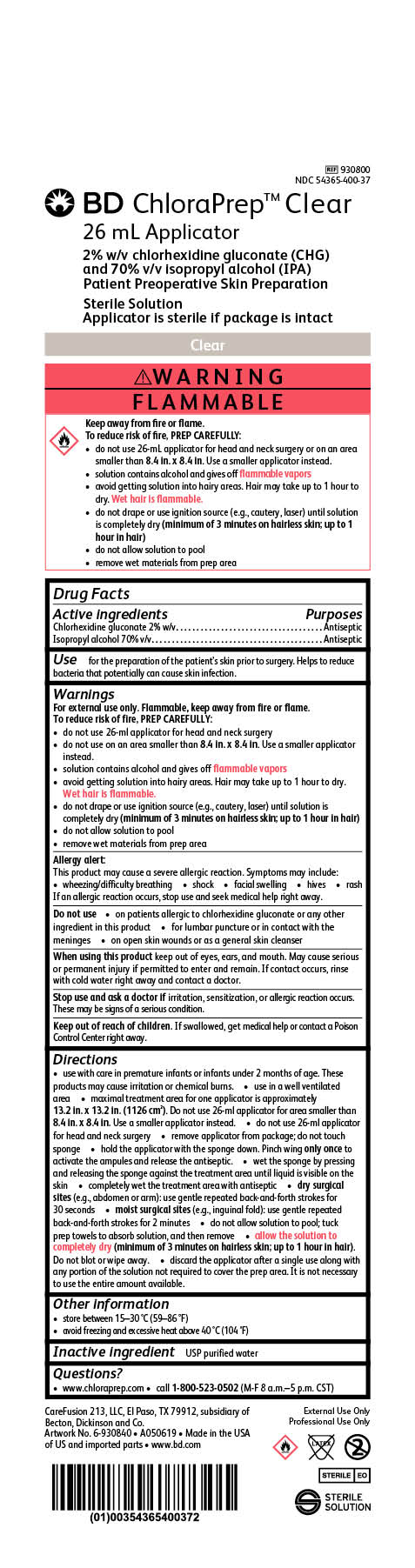
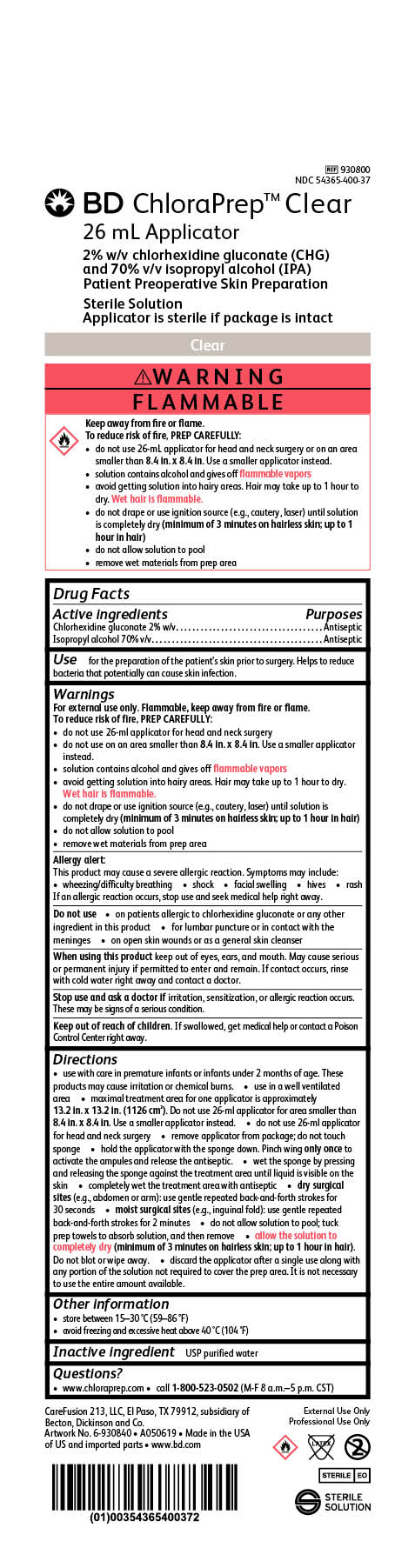
BD ChloraPrep Clear
26 mL Applicator
2% w/v chlorhexidine gluconate (CHG)
and 70% v/v Isopropyl alcohol (IPA)
Patient Preoperative Skin Preparation
Sterile Solution
Applicator is sterile if package is intact
REF 930800
NDC 54365-400-37
External Use Only
Professional Use Only
STERILE EO
STERILE SOLUTION
CareFusion 213, LLC, El Paso, TX 79912, subsidiary of Becton, Dickinson and Co.
Made in the USA of US and imported parts
www.bd.com
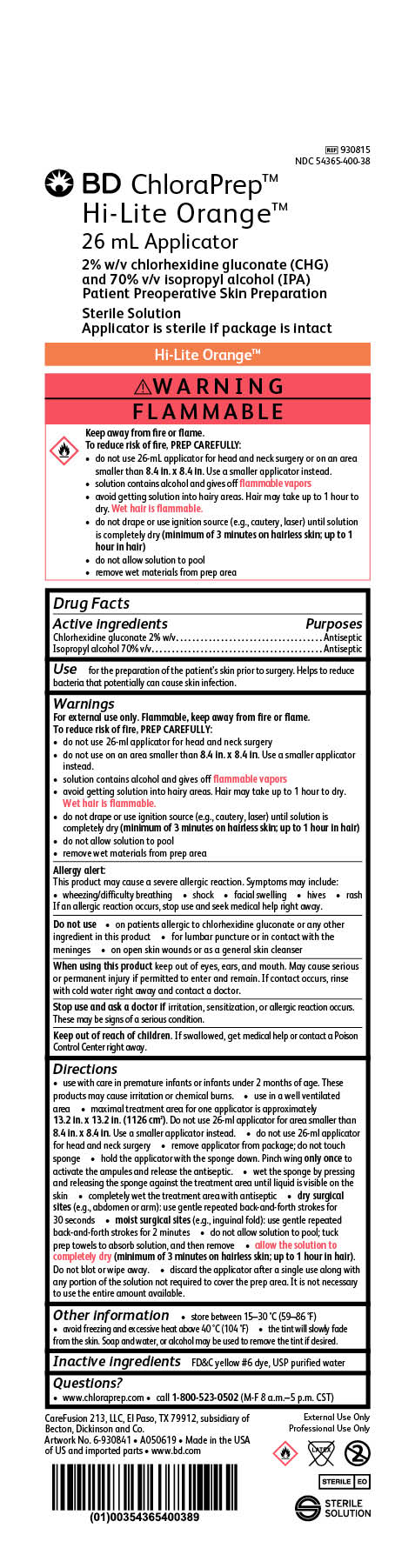
Package Label
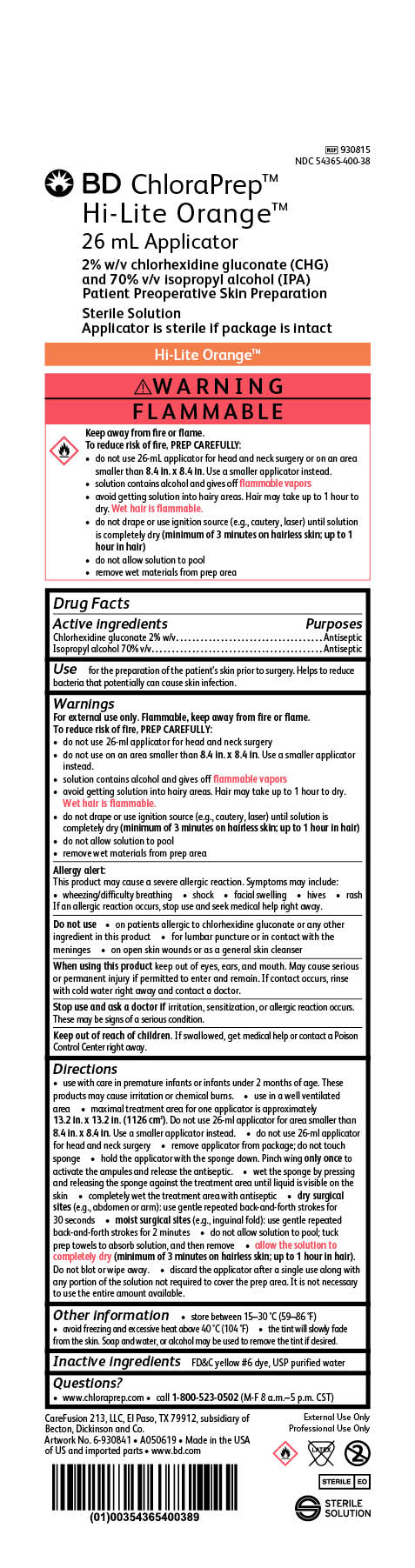
BD ChloraPrep Orange
26 mL Applicator
2% w/v chlorhexidine gluconate (CHG)
and 70% v/v Isopropyl alcohol (IPA)
Patient Preoperative Skin Preparation
Sterile Solution
Applicator is sterile if package is intact
REF 930815
NDC 54365-400-38
External Use Only
Professional Use Only
STERILE EO
STERILE SOLUTION
CareFusion 213, LLC, El Paso, TX 79912, subsidiary of Becton, Dickinson and Co.
Made in the USA of US and imported parts
www.bd.com
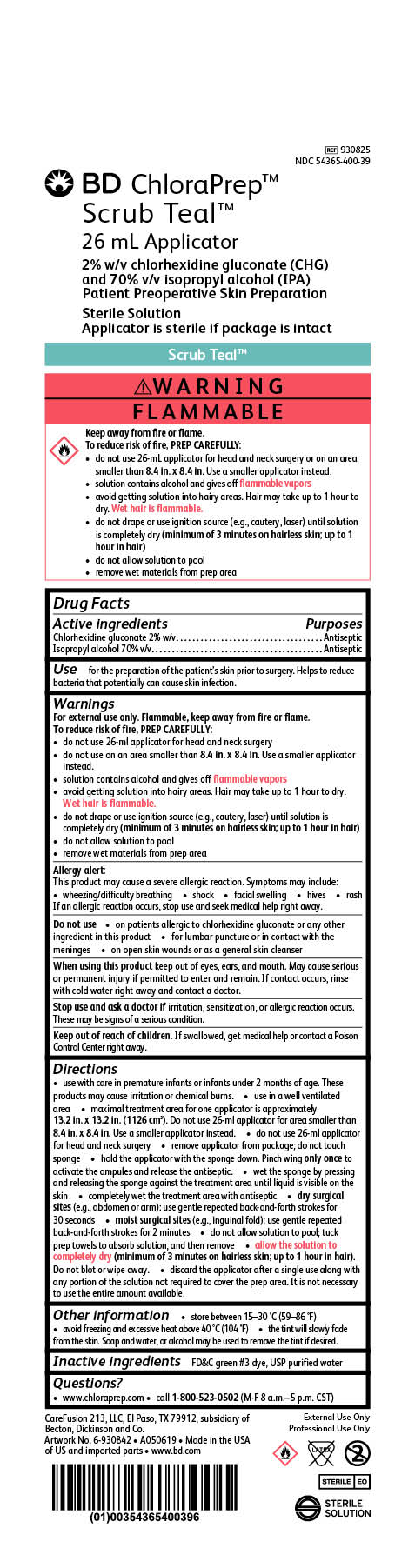
Package Label
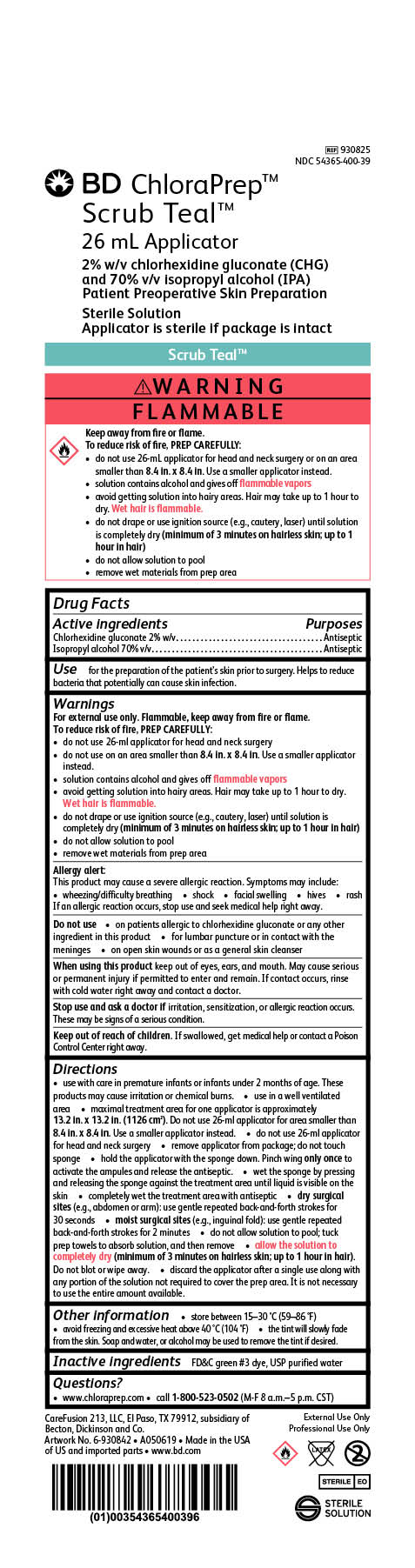
BD ChloraPrep Scrub Teal
26 mL Applicator
2% w/v chlorhexidine gluconate (CHG)
and 70% v/v Isopropyl alcohol (IPA)
Patient Preoperative Skin Preparation
Sterile Solution
Applicator is sterile if package is intact
REF 930825
NDC 54365-400-39
External Use Only
Professional Use Only
STERILE EO
STERILE SOLUTION
CareFusion 213, LLC, El Paso, TX 79912, subsidiary of Becton, Dickinson and Co.
Made in the USA of US and imported parts
www.bd.com
-
INGREDIENTS AND APPEARANCE
CHLORAPREP ONE-STEP
chlorhexidine gluconate and isopropyl alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54365-400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) (CHLORHEXIDINE - UNII:R4KO0DY52L) CHLORHEXIDINE GLUCONATE 20 mg in 1 mL ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54365-400-37 1 in 1 POUCH 05/24/2019 1 26 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 2 NDC:54365-400-38 1 in 1 POUCH 05/24/2019 2 26 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) 3 NDC:54365-400-39 1 in 1 POUCH 05/24/2019 3 26 mL in 1 APPLICATOR; Type 9: Other Type of Part 3 Combination Product (e.g., Drug/Device/Biological Product) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020832 05/24/2019 Labeler - CareFusion 213, LLC (826496312) Registrant - Becton, Dickinson and Company (832696038) Establishment Name Address ID/FEI Business Operations CareFusion 213, LLC 826496312 analysis(54365-400) , manufacture(54365-400) , label(54365-400) , pack(54365-400) , sterilize(54365-400)