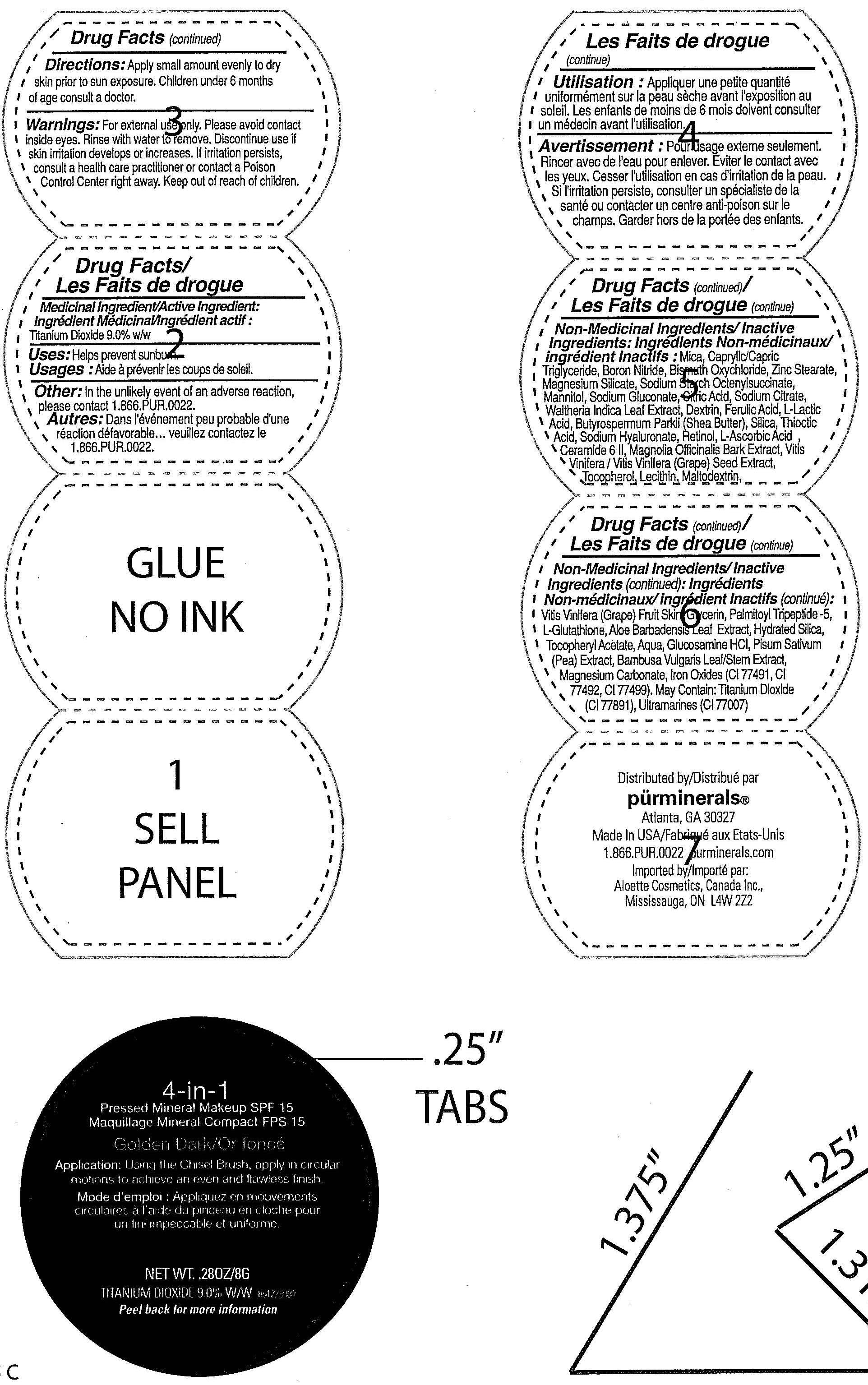
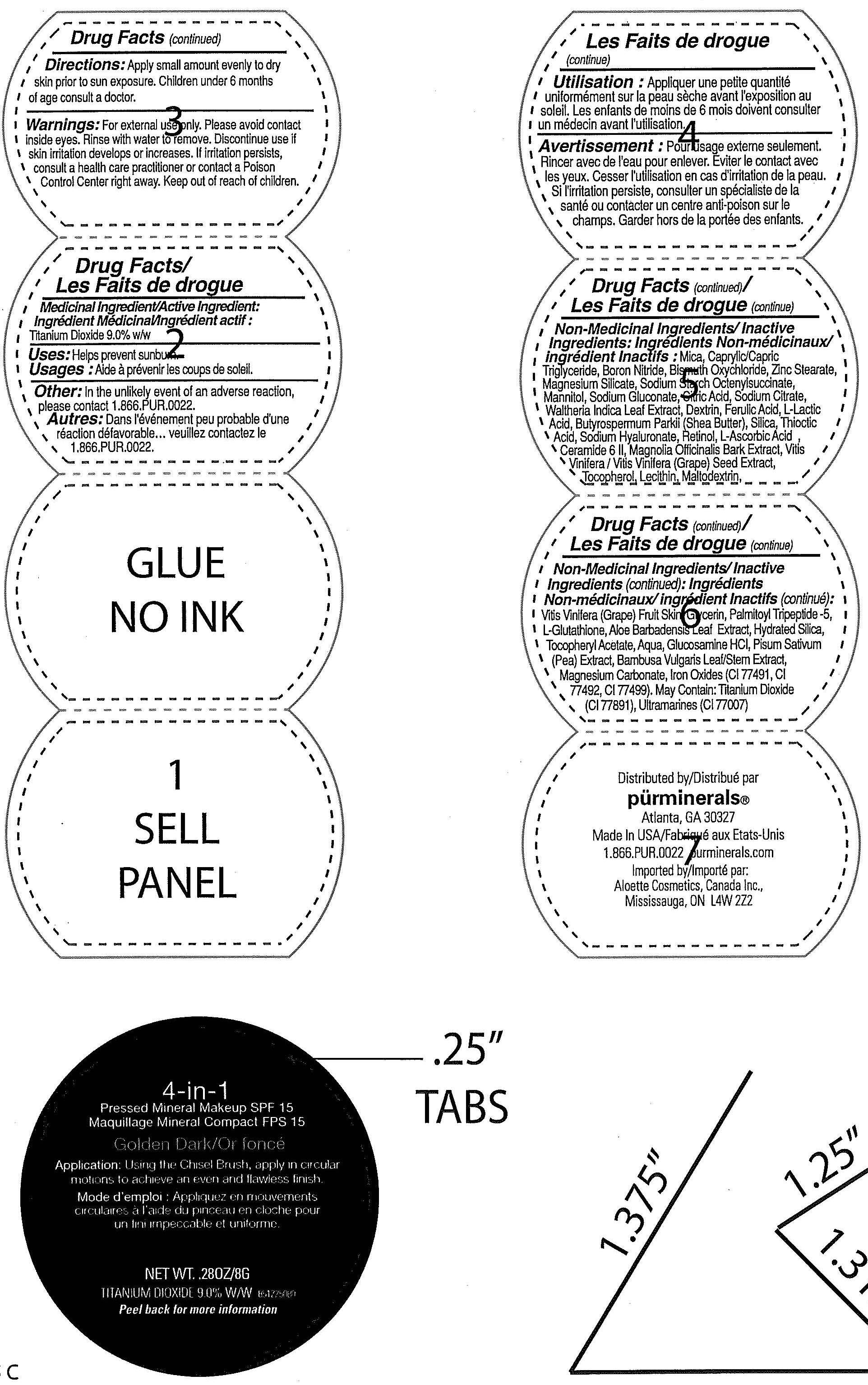
Label: 4 IN 1 PRESSED MINERAL SPF 15 GOLDEN DARK- titanium dioxide powder
-
Contains inactivated NDC Code(s)
NDC Code(s): 67345-0787-0 - Packager: PUR MINERALS
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 15, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
INACTIVE INGREDIENT
Inactive Ingredients : Mica , Boron Nitride , Caprylic/Capric Triglyceride , Bismuth Oxychloride , Zinc Stearate , Magnesium Silicate , Magnesium Carbonate ,Sodium Starch Octenylsuccinate, Mannitol,
Sodium Gluconate , Citric Acid, Sodium Citrate , Walthena Indica Leaf Extract , Dextrin , Ferulic Acid, L-Lactic Acid, Butyrospermum Parkii (Shea Butter) , Silica, Thioctic Acid,Sodium Hyarluronate, Retinol,
L-Ascorbic Acid, Ceramide 6 II , Magnolia Officinalis Bark Extract, Vitis Vinifera (Grape) Seed Extract , Tocopherol , Lecithin , Maltodextrin , Vitis Vinifera (Grape) Fruit Skin, Glycerin, Palmitoyl Tripeptide-5,
L-Glucothione, Aloe Barbadensis Leaf Extract, Hydrated Silica , Tocopheryl Acetate , Aqua, Glucosamine HCI, Pisum Sativum (Pea) Extract, Bambus Vulgaris Leaf/Stem Extract, Magnesium Carbonate ,
Iron oxide , May contain : Titanium Dioxide, Ultramarines.
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
4 IN 1 PRESSED MINERAL SPF 15 GOLDEN DARK
titanium dioxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:67345-0787 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 0.72 g in 8 g Inactive Ingredients Ingredient Name Strength Mica (UNII: V8A1AW0880) Boron Nitride (UNII: 2U4T60A6YD) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) Bismuth Oxychloride (UNII: 4ZR792I587) Zinc Stearate (UNII: H92E6QA4FV) Magnesium Silicate (UNII: 9B9691B2N9) MAGNESIUM CARBONATE (UNII: 0E53J927NA) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Shea Butter (UNII: K49155WL9Y) Acetate Ion (UNII: 569DQM74SC) Iron (UNII: E1UOL152H7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:67345-0787-0 8 g in 1 CASE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 09/15/2011 Labeler - PUR MINERALS (780516402)