Label: 4237 FIRST AID KIT kit
- NDC Code(s): 0498-0121-00, 0498-0143-04, 0498-0202-00, 0498-4237-01
- Packager: Honeywell Safety Products USA, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
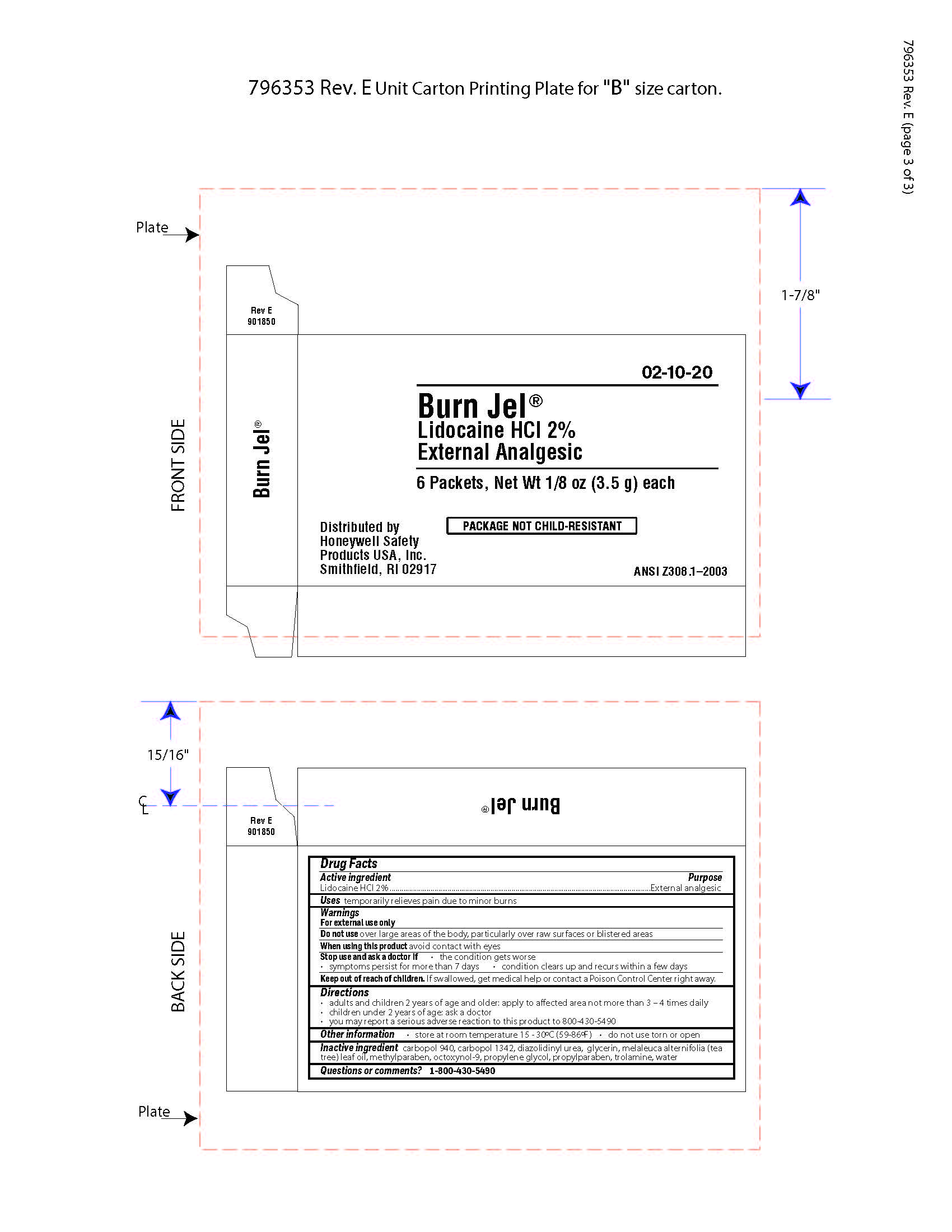
- Burn Jel Active ingredient
- Burn Jel Purpose
- Burn Jel Uses
- Burn Jel Warnings
- Burn Jel Keep out of reach of children
- Burn Jel Directions
- Burn Jel Other information
- Burn Jel Inactive ingredients
- Burn Jel Questions
- PVP Wipe Active ingredient
- PVP Wipe Purpose
- PVP Wipe Uses
- PVP Wipe Warnings
- PVP Wipe Keep out of reach of children
- PVP Wipe Directions
- PVP Wipe Other information
- PVP Wipe Inactive ingredients
- PVP Wipe Questions
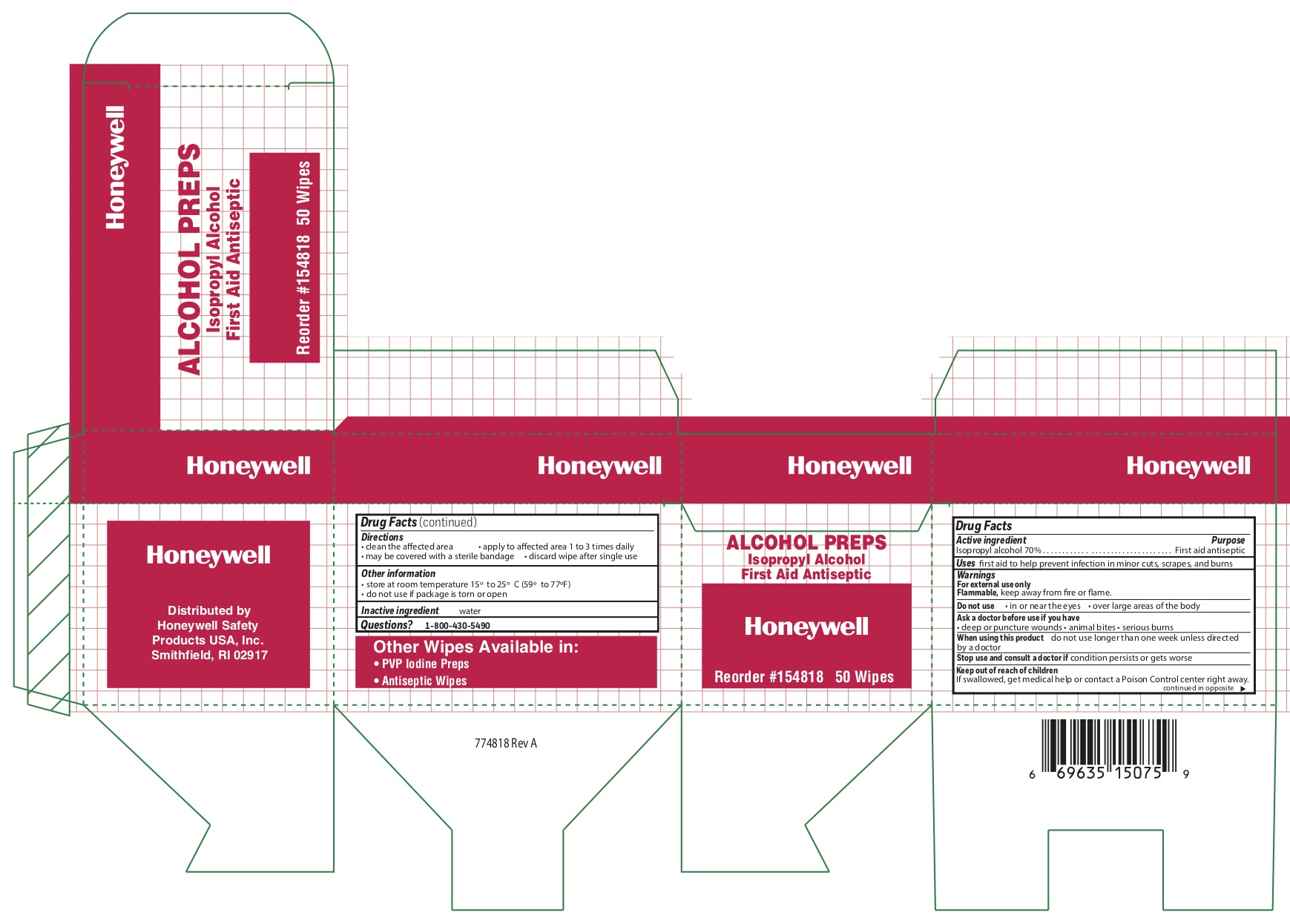
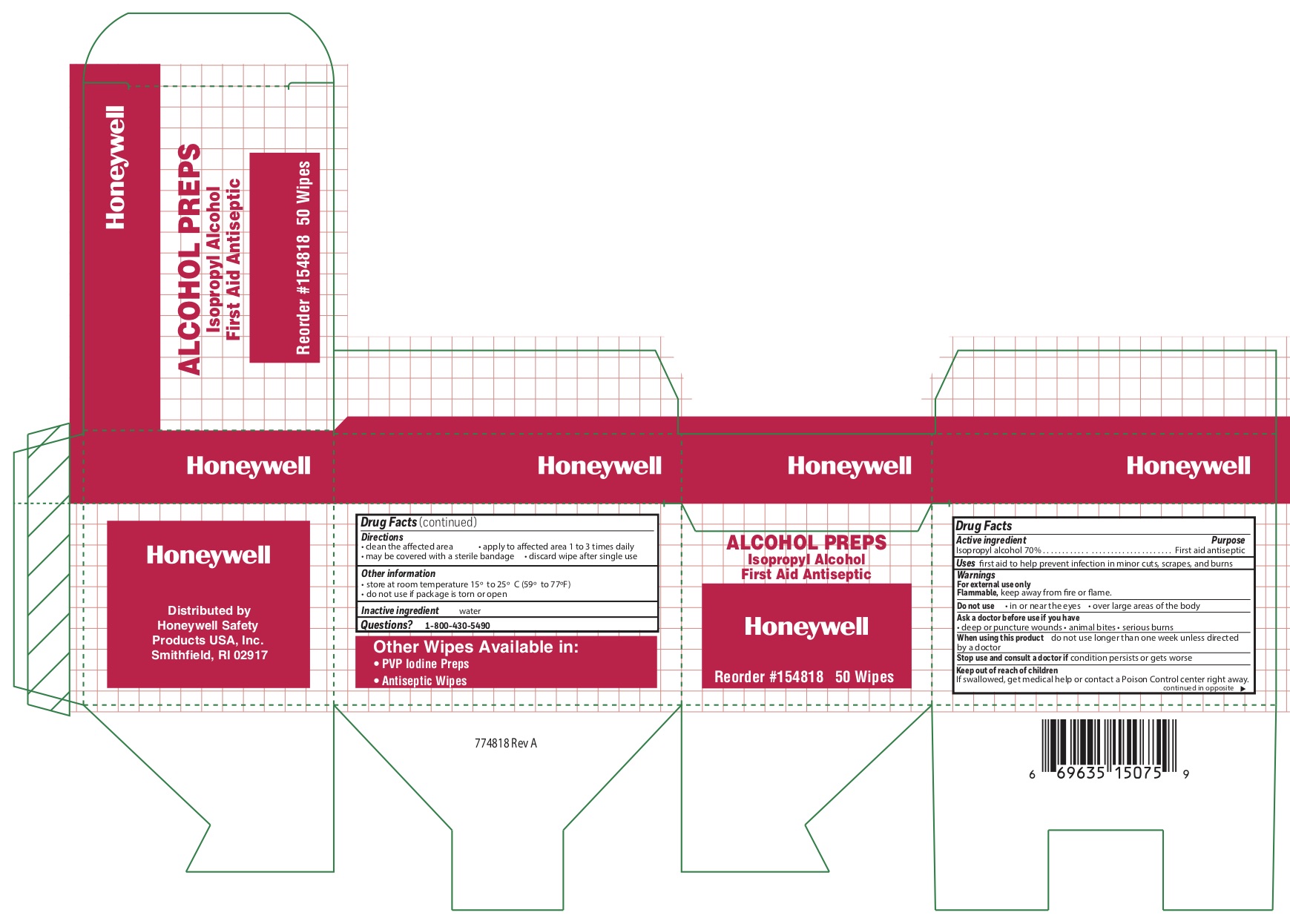
- Alcohol Active ingredient
- Alcohol Purpose
- Alcohol Uses
- Alcohol Warnings
- Alcohol Directions
- Alcohol Other information
- Alcohol Inactive ingredient
- Alcohol Questions
-
4237
6836ALPD3 Kit Contents
1 GAUZE BANDAGE, 2" X 6 YD,2 PER
2 INSTANT COLD PACK 4" X 6"
1 EYEWASH BOTTLES 1 OZ, UNITIZED 2/BX
1 BURN JEL 1/8 OZ, 6 PER
1 WATER JEL DRESSING 4" X 4"
1 ALCOHOL PREP PADS 10P
4 ADH BDG, CLOTH, 1"X3", 16 PER
1 FIRST AID GUIDE ASHI
1 SCISSOR UTILITY SHEARS 7-1/4"
1 ISO-SHIELD CPR ADULT/CHLD 1BG
LBL STOCK 6-3/8"X4"
1 LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 PICK-UP SCOOP W/SCRAPER
1 WATER-JEL BURN DRESSING 2 X 6
1 KIT STL 36 UN WHT 01 HOR SHELF
1 KNUCKLE BANDS 16'S
1 ADHS TAPE .5"X2.5YD 2
1 FINGERTIP "T" 8/BX
1 PVP IODINE SWABS 10
1 GAUZE PADS 3"X3" 4/BX
1 SCISSOR & FORCEP 1 EA
1 RESCUE BLANKET 1EA
1 RED BIO BAGS 2/BX
1 FACE MASK/EYE SHIELD
1 LIQD TRTMNT SYS 1 EA
1 DISP. TOWEL/WIPES 2EA
3 NITRILE GLOVES 1 PR
- Burn Jel Principal Display Panel
- PVP Wipe Principal Display Panel
- Alcohol Principal Display Panel
- 4237 Kit Label 6836ALPD3
-
INGREDIENTS AND APPEARANCE
4237 FIRST AID KIT
4237 first aid kit kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0498-4237 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-4237-01 1 in 1 KIT 09/12/2018 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 6 PACKET 21 mL Part 2 20 POUCH 6 mL Part 3 10 POUCH 4 mL Part 1 of 3 BURN JEL
gel for burns gelProduct Information Item Code (Source) NDC:0498-0202 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 2 g in 100 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) PROPYLPARABEN (UNII: Z8IX2SC1OH) GLYCERIN (UNII: PDC6A3C0OX) TEA TREE OIL (UNII: VIF565UC2G) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) TROLAMINE (UNII: 9O3K93S3TK) METHYLPARABEN (UNII: A2I8C7HI9T) OCTOXYNOL 9 (UNII: 7JPC6Y25QS) DIPROPYLENE GLYCOL (UNII: E107L85C40) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0202-00 3.5 mL in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/08/2017 Part 2 of 3 PVP IODINE WIPE
povidone-iodine 10% swabProduct Information Item Code (Source) NDC:0498-0121 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POVIDONE-IODINE (UNII: 85H0HZU99M) (IODINE - UNII:9679TC07X4) IODINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength NONOXYNOL-9 (UNII: 48Q180SH9T) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0121-00 0.3 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Part 3 of 3 ALCOHOL WIPE
isopropyl alcohol swabProduct Information Item Code (Source) NDC:0498-0143 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0498-0143-04 0.4 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/18/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 09/12/2018 Labeler - Honeywell Safety Products USA, Inc. (118768815)