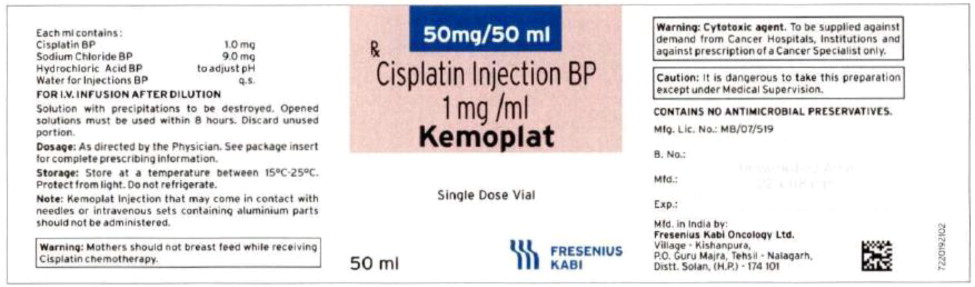
Label: CISPLATIN injection, solution
- NDC Code(s): 65219-359-50
- Packager: Fresenius Kabi USA, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HEALTH CARE PROVIDER LETTER
Fresenius Kabi USA, LLC
Three Corporate Drive
Lake Zurich, Illinois 60047
T 847-550-2300
T 888-391-6300August 30, 2023 www.fresenius-kabi.com/us
IMPORTANT PRESCRIBING INFORMATION Subject: Temporary Importation of CISplatin Injection to Address Drug Shortage Dear Healthcare Professional,
Due to the critical shortage of CISplatin Injection in the U.S. market, Fresenius Kabi USA, LLC (Fresenius Kabi USA) is coordinating with the U.S. Food and Drug Administration (FDA) to increase availability of the drug. Fresenius Kabi USA has initiated temporary importation of CISplatin Injection BP (KEMOPLAT) 50 mg/50 mL into the U.S. market. This product is marketed in Europe and is manufactured in India and is not FDA-approved.
At this time, no other entity except Fresenius Kabi USA is authorized by the FDA to import or distribute Fresenius Kabi's CISplatin Injection in the U.S.
Effective immediately, and during this temporary period, Fresenius Kabi USA will distribute the following presentation of CISplatin Injection to address the critical shortage:
Product Name Quantity Description U.S. NDC Number Lot Number Exp Date CISplatin Injection BP†
(KEMOPLAT)
50 mg/50 mL
(1 mg/mL)
†BP = British
Pharmacopeia1 amber single dose vial per carton Clear, colorless to pale yellow solution. Each mL contains 1 mg Cisplatin and 9 mg sodium chloride in water for injection 65219-359-50 87230281A 05/2025 It is important to note the following:
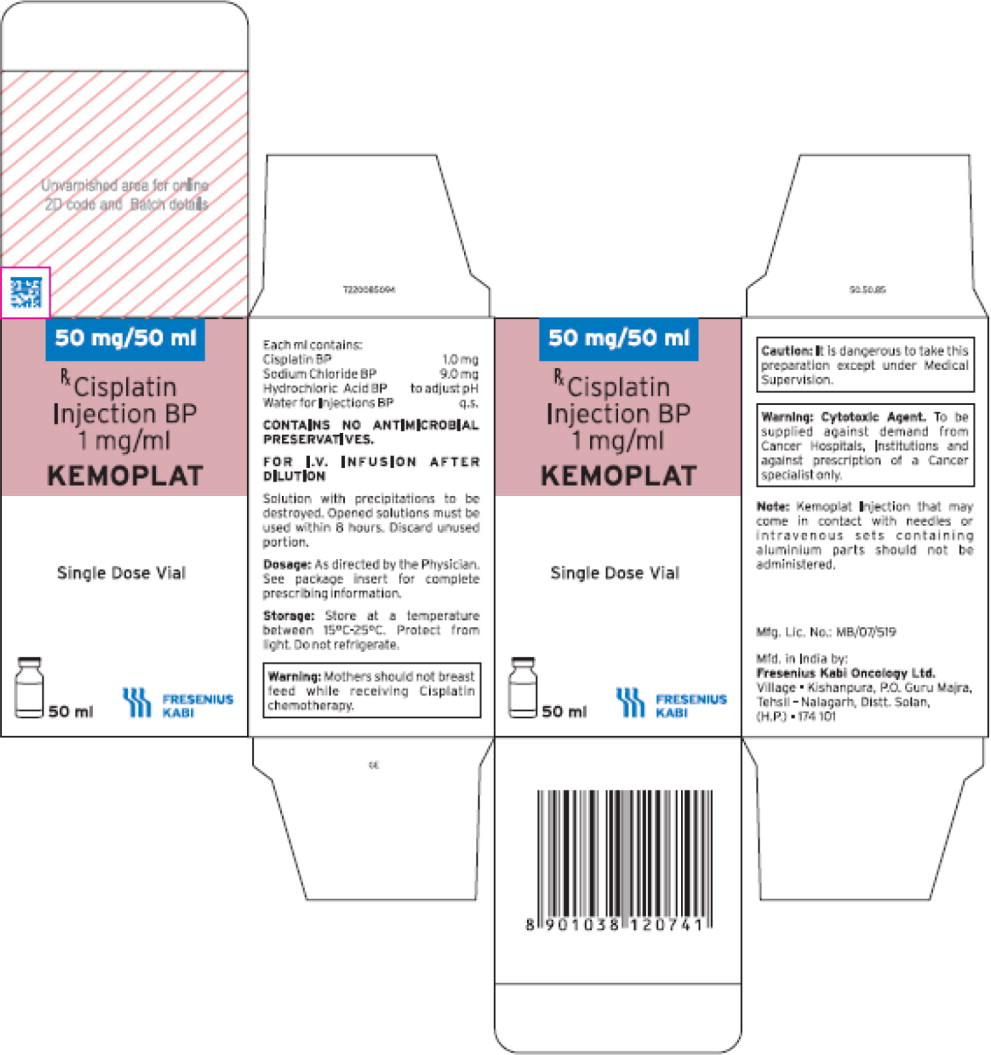
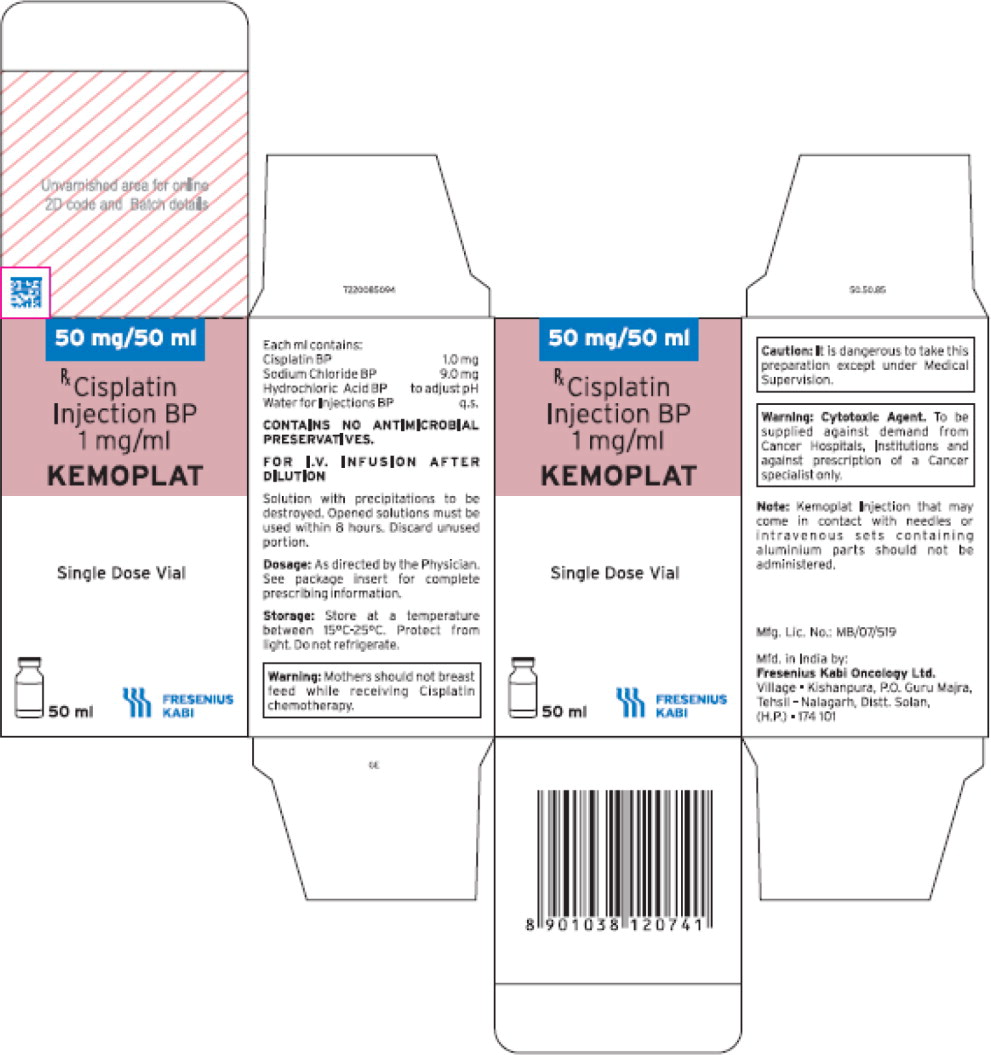
- The imported product is labeled CISplatin Injection BP (KEMOPLAT) 50 mg/50 mL (1 mg/mL). BP stands for British Pharmacopeia and is not an acronym for an ingredient in the formulation. The BP provides quality standards for UK pharmaceutical substances and medicinal products.
- The carton labeling and container labeling do not include the warning statements, “Stop! Verify Drug Name and Dose!” or “CISplatin doses greater than 100 mg/m2 once every 3 to 4 weeks are rarely used.” See U.S. package insert.
- The product is a single dose vial. Fresenius Kabi does NOT have extended stability data once the vial is punctured or information on withdrawing multiple doses.
- The imported product is a clear, colorless to pale yellow solution while the US product is a clear, colorless solution.
- Any barcodes on CISplatin Injection will not be appropriately recognized by scanning systems used in the United States and should NOT be used. Institutions should manually input the product into their systems to confirm that barcode systems do not provide incorrect information when the product is scanned. Alternative procedures should be followed to assure that the correct drug product is being prepared and administered to individual patients.
- In addition, the carton of the imported product does not include a product identifier as required under the Drug Supply Chain Security Act (DSCSA). Specifically, each package of product does not include the NDC, unique serial number, lot number, and expiration date in both human-readable form and a two-dimensional data matrix barcode.
Please ensure that staff and others within the institution who may be involved in the administration of CISplatin Injection receive a copy of this letter and review the information.
This communication and product information is available on the Fresenius Kabi USA website: https://www.fresenius-kabi.com/us/documents/CISplatin-DHCP-Letter.pdf as well as on the FDA Drug Shortage website http://www.fda.gov/Druqs/DruqSafetv/DruqShortaqes/default.htm.
REPORTING ADVERSE EVENTS
To report adverse events experienced with the use of this product, call Fresenius Kabi USA Vigilance at 1-800-551-7176, Monday - Friday, between the hours of 8 a.m. and 5 p.m. (CST), or e-mail adverse.events.USA@fresenius-kabi.com.
To report a product quality complaint with the use of this product, call 1-800-551-7176 or e-mail productcomplaint.USA@fresenius-kabi.com.
Fresenius Kabi USA CONTACT NUMBERS: Please use the following contact numbers as appropriate:
Reason To Call Department Number ADE Reporting Vigilance Department 1-800-551-7176 Clinical/Technical Info. Or Product Quality Complaint Medical Affairs Department Product Availability & Ordering Customer Service Department 1-888-386-1300 Adverse reactions or quality problems experienced with the use of this product may be reported to the FDA's MedWatch Adverse Event Reporting program either online, by regular mail or by fax.
- Complete and submit the report Online: www.fda.gov/medwatch/report.htm
- Regular Mail or Fax: Download form http://www.fda.gov/medwatch/qetforms.htm or call 1-800-332-1088 to request a reporting form, then complete and return to the address on the pre-addressed form, or submit by fax to 1-800-FDA-0178 (1-800-332-0178).
Sincerely,
Anthony Giessert, Ph.D.
Vice President, Quality Assurance
Fresenius Kabi USA, LLCCISplatin Injection Product Labels
†BP = British Pharmacopeia
U.S. FDA Approved Product Imported Product† Carton Label 

Vial Label 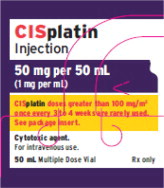
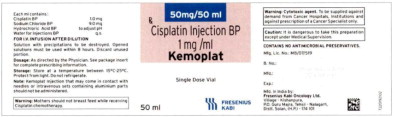
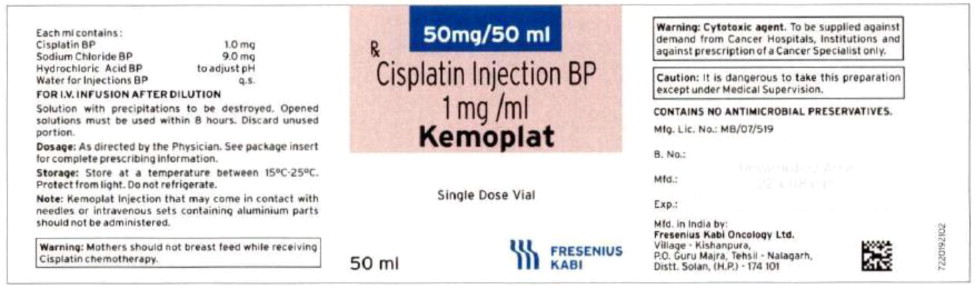
Product Image 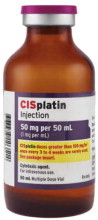
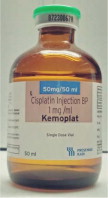
CISplatin Injection Product and Prescribing Information Side-by-Side Comparison Table
U.S. FDA Approved Product Imported Product Product Name CISplatin Injection CISplatin Injection BP (KEMOPLAT) (BP = British Pharmacopeia) Active Ingredient CISplatin CISplatin Available
Strengths/Concentrations50 mg/50 mL (1 mg/mL)
100 mg/100 mL (1 mg/mL)
200 mg/200 mL (1 mg/mL)50 mg/50 mL (1 mg/mL) Route of Administration For Intravenous Use (must be further diluted prior to administration) For I.V. Infusion After Dilution Container Amber multiple dose vial with 28 mm vial closure
The container closure is not made with natural rubber latex.Amber single dose vial with 20 mm vial closure
The container closure is not made with natural rubber latex.Product Description Cisplatin Injection is a clear, colorless, sterile aqueous solution. Each 50 mL, 100 mL or 200 mL amber vial of Cisplatin Injection contains: 1 mg/mL cisplatin, 9 mg/mL sodium chloride, hydrochloric acid and/or sodium hydroxide to adjust pH, and water for injection to a final volume of 50 mL, 100 mL or 200 mL, respectively. The pH range of Cisplatin Injection is 3.8 to 5.9. Kemoplat is a clear, colourless to pale yellow solution. KEMOPLAT is a sterile solution of Cisplatin BP 1.0mg/ml (50ml pack), sodium chloride BP
9mg/ml in Water for Injections BP.
Storage and Handling Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Do not refrigerate. Protect from light.
Stability
Cisplatin is a sterile, multiple dose vial without preservatives. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Do not refrigerate. Protect unopened container from light. The cisplatin remaining in the amber vial following initial entry is stable for 28 days protected from light or for 7 days under fluorescent room light.Store at a temperature between 15°C - 25°C. Protect from light. Do not refrigerate.
Indications Cisplatin Injection is indicated as therapy to be employed as follows:
Metastatic Testicular Tumors
In established combination therapy with other approved chemotherapeutic agents in patients with metastatic testicular tumors who have already received appropriate surgical and/or radiotherapeutic procedures.
Metastatic Ovarian Tumors
In established combination therapy with other approved chemotherapeutic agents in patients with meta static ovarian tumors who have already received appropriate surgical and/or radiotherapeutic procedures. An established combination consists of cisplatin and cyclophosphamide. Cisplatin Injection, as a single agent, is indicated as secondary therapy in patients with metastatic ovarian tumors refractory to standard chemotherapy who have not previously received Cisplatin Injection therapy.
Advanced Bladder Cancer
Cisplatin Injection is indicated as a single agent for patients with transitional cell bladder cancer which is no longer amenable to local treatments, such as surgery and/or radiotherapy.KEMOPLAT is indicated for following indications:
Metastatic Testicular Cancer: In established combination therapy with other approved chemotherapeutic agents in patients with metastatic testicular tumors who have already received appropriate surgical and/or radio therapeutic procedures. Metastatic Ovarian Cancer: In established combination therapy with other approved chemotherapeutic agents KEMOPLAT is used in patients with metastatic ovarian tumors who have already received appropriate surgical and/or radio therapeutic procedures. Also as a single agent, it is indicated as secondary therapy in patients with metastatic ovarian tumors refractory to standard chemotherapy who have not previously received cisplatin therapy.
Advanced Bladder Cancer: Cisplatin is indicated as a single agent for patients with transitional cell bladder cancer, which is no longer amenable to local treatments such as surgery and/or radiotherapy.
Contraindications Cisplatin is contraindicated in patients with pre-existing renal impairment. Cisplatin should not be employed in myelosuppressed patients, or in patients with hearing impairment.
Cisplatin is contraindicated in patients with a history of allergic reactions to cisplatin or other platinum-containing compounds.Use of cisplatin is contraindicated in patients with a history of hypersensitivity to cisplatin or other platinum containing compounds. Cisplatin should not be used in patients with preexisting renal impairment, myelosuppressed patients or patients with hearing impairment.
Cisplatin is administered by slow intravenous infusion. CISPLATIN SHOULD NOT BE GIVEN BY RAPID INTRAVENOUS INJECTION. Note: Needles or intravenous sets containing aluminum parts that may come in contact with cisplatin should not be used for preparation or administration. Aluminum reacts with cisplatin, causing precipitate formation and a loss of potency.
Metastatic Testicular Tumors The usual cisplatin dose for the treatment of testicular cancer in combination with other approved chemo therapeutic agents is 20 mg/m2 IV daily for 5 days per cycle.
Metastatic Ovarian Tumors The usual cisplatin dose for the treatment of metastatic ovarian tumors in combination with cyclophosphamide is 75 to 100 mg/m2 IV per cycle once every 4 weeks (DAY 1). The dose of cyclophosphamide when used in combination with cisplatin is 600 mg/m2 IV once every 4 weeks (DAY 1). For directions for the administration of cyclo-phosphamide, refer to the cyclophosphamide package insert. In combination therapy, cisplatin and cyclophosphamide are administered sequentially. As a single agent, cisplatin should be administered at a dose of 100 mg/m2 IV per cycle once every 4 weeks.
Advanced Bladder Cancer Cisplatin should be administered as a single agent at a dose of 50 to 70 mg/m2 IV per cycle once every 3 to 4 weeks depending on the extent of prior exposure to radiation therapy and/or prior chemotherapy. For heavily pre-treated patients an initial dose of 50 mg/m2 per cycle repeated every 4 weeks is recommended.
All Patients
Pretreatment hydration with 1 to 2 liters of fluid infused for 8 to 12 hours prior to a cisplatin dose is recommended. The drug is then diluted in 2 liters of 5% Dextrose in 1/2 or 1/3 normal saline containing 37.5 g of mannitol, and infused over a 6- to 8-hour period. If diluted solution is not to be used within 6 hours, protect solution from light. Do not dilute cisplatin in just 5% Dextrose Injection. Adequate hydration and urinary output must be maintained during the following 24 hours.
A repeat course of cisplatin should not be given until the serum creatinine is below 1.5 mg/100 mL, and/or the BUN is below 25 mg/100 mL. A repeat course should not be given until circulating blood elements are at an acceptable level (platelets ≥100,000/mm3, WBC ≥4,000/mm3). Subsequent doses of cisplatin should not be given until an audiometric analysis indicates that auditory acuity is within normal limits. Preparation of Intravenous Solutions The aqueous solution should be used intravenously only and should be administered by IV infusion over a 6- to 8-hour period (see DOSAGE AND ADMINISTRATION). Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
NOTE TO PHARMACIST: Exercise
caution to prevent inadvertent cisplatin overdosage. Please call prescriber if dose is greater than 100 mg/m2 per cycle. Aluminum and flip-off seal of vial have been imprinted with the following statement: CALL DR. IF DOSE >100 MG/M2/CYCLE.Metastatic Testicular Cancer: The usual dose for the treatment of testicular cancer in combination with other approved chemotherapeutic agents is 20 mg/m2 I.V. daily for 5 days per cycle.
Metastatic Ovarian Cancer: The usual cisplatin dose for the treatment of metastatic ovarian cancer in combination with cyclophosphamide is 75-100 mg/m2 I.V. per cycle once every 4 weeks, (Day 1).
As a single agent cisplatin should be administered as 100 mg/m2/cycle IV every 4 weeks.
Advanced Bladder Cancer: cisplatin should be administered as a single agent at a dose of 50-70 mg/m2 I.V. per cycle once every 3 to 4 weeks depending on the extent of prior exposure to radiation therapy and/or prior chemotherapy. For heavily pretreated patients an initial dose of 50 mg/m2 per cycle repeated every four weeks is recommended.
a) Pre Treatment Hydration: Patients should be adequately hydrated before and for 24 hrs. after KEMOPLAT administration in order to induce diuresis and minimize nephrotoxicity. Hydration may be achieved either by I.V. infusion of 2 liters of 0.9% sodium chloride or dextrose saline (Dextrose 5% in one fifth normal saline (0.18% Sodium chloride injection) over a 6-12 hr. period. During the last 30 minutes of the pre treatment hydration or after the hydration, 375 ml of 20% mannitol injection may be administered via a side arm drip.
b) Preparation of KEMOPLAT infusion: KEMOPLAT should be added to 2 liters of 0.9% sodium chloride injection or Dextrose-Saline solution.
c) Treatment:
Following pre-hydration, KEMOPLAT infusion is administered over 1-2 hrs. A longer infusion time of 6-8 hrs may decrease gastrointestinal and renal toxicities.
d) Post Treatment Hydration
It is recommended that I.V. hydration should continue after treatment with administration of 2 liters 0.9% sodium chloride IV infusion or Dextrose-saline over a period of 6-12 hours.
NOTE: Since aluminum reacts with and inactivates cisplatin, it is important not to use needles or other equipment that contain aluminum while preparing or administering KEMOPLAT.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CISPLATIN
cisplatin injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65219-359 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CISPLATIN (UNII: Q20Q21Q62J) (CISPLATIN - UNII:Q20Q21Q62J) CISPLATIN 1 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65219-359-50 1 in 1 CARTON 09/01/2023 02/22/2024 1 50 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 09/01/2023 Labeler - Fresenius Kabi USA, LLC (013547657) Establishment Name Address ID/FEI Business Operations Fresenius Kabi Oncology Limited 915786944 API MANUFACTURE(65219-359)