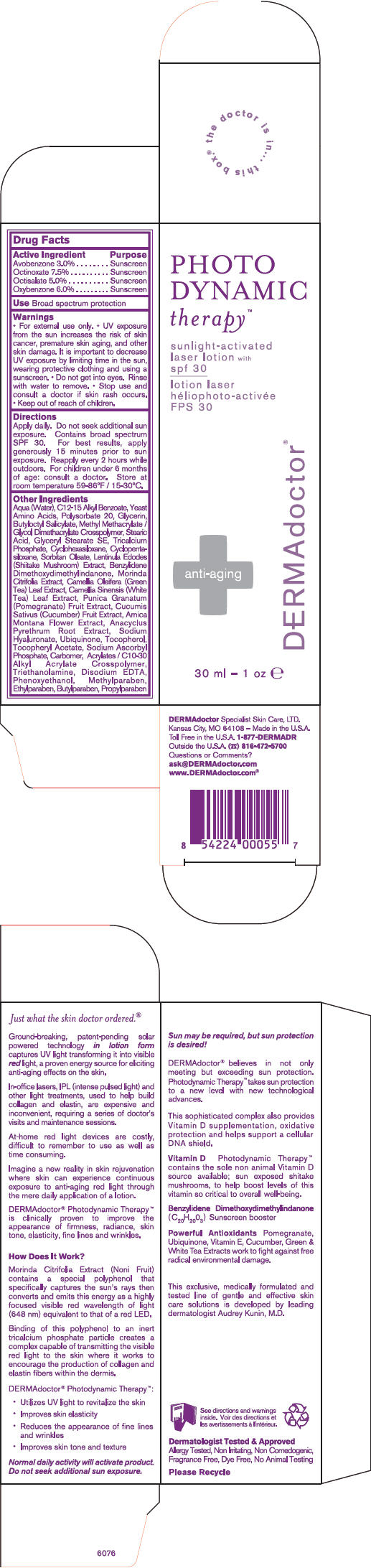
Label: PHOTO DYNAMIC THERAPY SPF 30- avobenzone, octinoxate, octisalate, and oxybenzone lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 65113-2432-1 - Packager: G.S. COSMECEUTICAL USA, INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 9, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
- Warnings
- Directions
-
Other Ingredients
Aqua (Water), C12-15 Alkyl Benzoate, Yeast Amino Acids, Polysorbate 20, Glycerin, Butyloctyl Salicylate, Methyl Methacrylate / Glycol Dimethacrylate Crosspolymer, Stearic Acid, Glyceryl Stearate SE, Tricalcium Phosphate, Cyclohexasiloxane, Cyclopentasiloxane, Sorbitan Oleate, Lentinula Edodes (Shiitake Mushroom) Extract, Benzylidene Dimethoxydimethylindanone, Morinda Citrifolia Extract, Camellia Oleifera (Green Tea) Leaf Extract, Camellia Sinensis (White Tea) Leaf Extract, Punica Granatum (Pomegranate) Fruit Extract, Cucumis Sativus (Cucumber) Fruit Extract, Arnica Montana Flower Extract, Anacyclus Pyrethrum Root Extract, Sodium Hyaluronate, Ubiquinone, Tocopherol, Tocopheryl Acetate, Sodium Ascorbyl Phosphate, Carbomer, Acrylates / C10-30 Alkyl Acrylate Crosspolymer, Triethanolamine, Disodium EDTA, Phenoxyethanol, Methylparaben, Ethylparaben, Butylparaben, Propylparaben
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL - 30 ml Carton
-
INGREDIENTS AND APPEARANCE
PHOTO DYNAMIC THERAPY SPF 30
avobenzone, octinoxate, octisalate, and oxybenzone lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65113-2432 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 6 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) C12-15 ALKYL BENZOATE (UNII: A9EJ3J61HQ) YEAST (UNII: 3NY3SM6B8U) AMINO ACIDS (UNII: 0O72R8RF8A) POLYSORBATE 20 (UNII: 7T1F30V5YH) GLYCERIN (UNII: PDC6A3C0OX) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) STEARIC ACID (UNII: 4ELV7Z65AP) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) TRICALCIUM PHOSPHATE (UNII: K4C08XP666) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) CYCLOMETHICONE 6 (UNII: XHK3U310BA) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) SHIITAKE MUSHROOM (UNII: 1A64QN2D2F) CAMELLIA OLEIFERA LEAF (UNII: 5077EL0C60) GREEN TEA LEAF (UNII: W2ZU1RY8B0) POMEGRANATE (UNII: 56687D1Z4D) CUCUMBER (UNII: YY7C30VXJT) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) ANACYCLUS PYRETHRUM ROOT (UNII: X820IRW34J) HYALURONATE SODIUM (UNII: YSE9PPT4TH) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) TROLAMINE (UNII: 9O3K93S3TK) EDETATE DISODIUM (UNII: 7FLD91C86K) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) BUTYLPARABEN (UNII: 3QPI1U3FV8) PROPYLPARABEN (UNII: Z8IX2SC1OH) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) Product Characteristics Color YELLOW (Off white to Light yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65113-2432-1 30 mL in 1 BOTTLE, PUMP Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 10/01/2010 Labeler - G.S. COSMECEUTICAL USA, INC. (017014734) Establishment Name Address ID/FEI Business Operations G.S. COSMECEUTICAL USA, INC. 017014734 MANUFACTURE