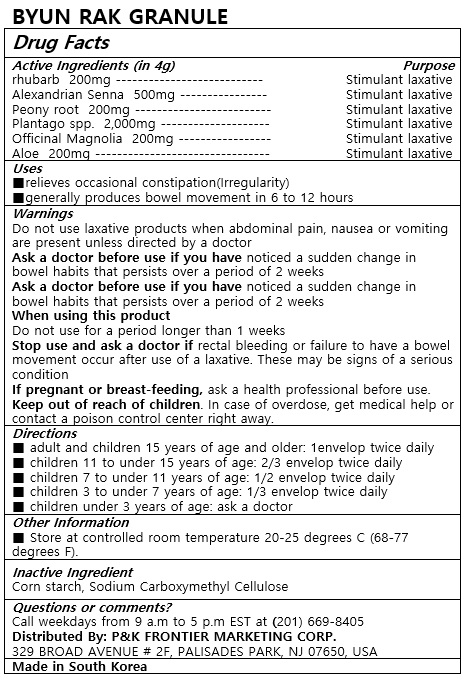
Label: BYUN RAK- rhubarb, alexandrian senna, peony root, plantago spp., officinal magnolia, magnoliae cortex, aloe granule
-
Contains inactivated NDC Code(s)
NDC Code(s): 72689-0043-1 - Packager: OASIS TRADING
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 21, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
-
INDICATIONS & USAGE
■ adult and children 15 years of age and older: 1envelop twice daily
■ children 11 to under 15 years of age: 2/3 envelop twice daily
■ children 7 to under 11 years of age: 1/2 envelop twice daily
■ children 3 to under 7 years of age: 1/3 envelop twice daily
■ children under 3 years of age: ask a doctor -
WARNINGS
Do not use laxative products when abdominal pain, nausea or vomiting are present unless directed by a doctor
Ask a doctor before use if you have noticed a sudden change in bowel habits that persists over a period of 2 weeks
Ask a doctor before use if you have noticed a sudden change in bowel habits that persists over a period of 2 weeks
When using this product
Do not use for a period longer than 1 weeks
Stop use and ask a doctor if rectal bleeding or failure to have a bowel movement occur after use of a laxative. These may be signs of a serious condition
If pregnant or breast-feeding, ask a health professional before use.
keep out of reach of children. In case of overdose, get medical help or contact a poison contral center right away. - INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BYUN RAK
rhubarb, alexandrian senna, peony root, plantago spp., officinal magnolia, magnoliae cortex, aloe granuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72689-0043 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SENNA LEAF (UNII: AK7JF626KX) (SENNA LEAF - UNII:AK7JF626KX) SENNA LEAF 500 mg in 4 g ALOE (UNII: V5VD430YW9) (ALOE - UNII:V5VD430YW9) ALOE 200 mg in 4 g MAGNOLIA OFFICINALIS BARK (UNII: 5M609NV974) (MAGNOLIA OFFICINALIS BARK - UNII:5M609NV974) MAGNOLIA OFFICINALIS BARK 200 mg in 4 g PAEONIA LACTIFLORA ROOT (UNII: 3Z3866YW6P) (PAEONIA LACTIFLORA ROOT - UNII:3Z3866YW6P) PAEONIA LACTIFLORA ROOT 200 mg in 4 g RHUBARB (UNII: G280W4MW6E) (RHUBARB - UNII:G280W4MW6E) RHUBARB 200 mg in 4 g PLANTAGO INDICA SEED COAT (UNII: I93093PU3S) (PLANTAGO INDICA SEED COAT - UNII:I93093PU3S) PLANTAGO INDICA SEED COAT 2000 mg in 4 g Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) STARCH, CORN (UNII: O8232NY3SJ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72689-0043-1 20 in 1 BOX 11/13/2018 1 4 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/13/2018 Labeler - OASIS TRADING (689991468) Registrant - OASIS TRADING (689991468) Establishment Name Address ID/FEI Business Operations OASIS TRADING 689991468 manufacture(72689-0043) , label(72689-0043)