Label: CHILDRENS ALLERGY- fexofenadine hydrochloride suspension
- NDC Code(s): 61269-527-94, 61269-527-98
- Packager: H2-Pharma, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 20, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
-
Directions
- shake well before using
- use only with enclosed dosing cup
Note: mL = milliliters adults and children 12 years of age and over take 10 mL every 12 hours; do not take more than 20 mL in 24 hours children 2 to under 12 years of age take 5 mL every 12 hours; do not take more than 10 mL in 24 hours children under 2 years of age ask a doctor adults 65 years of age and older ask a doctor consumers with kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
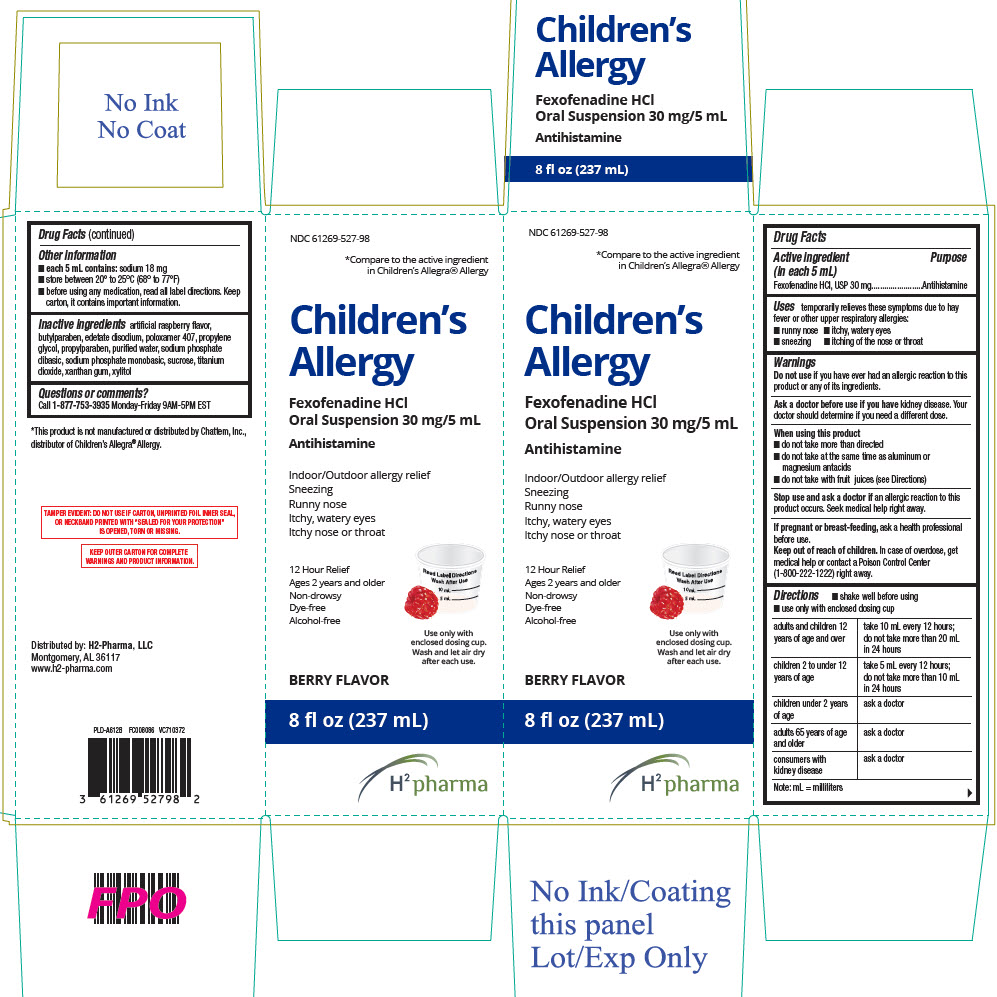
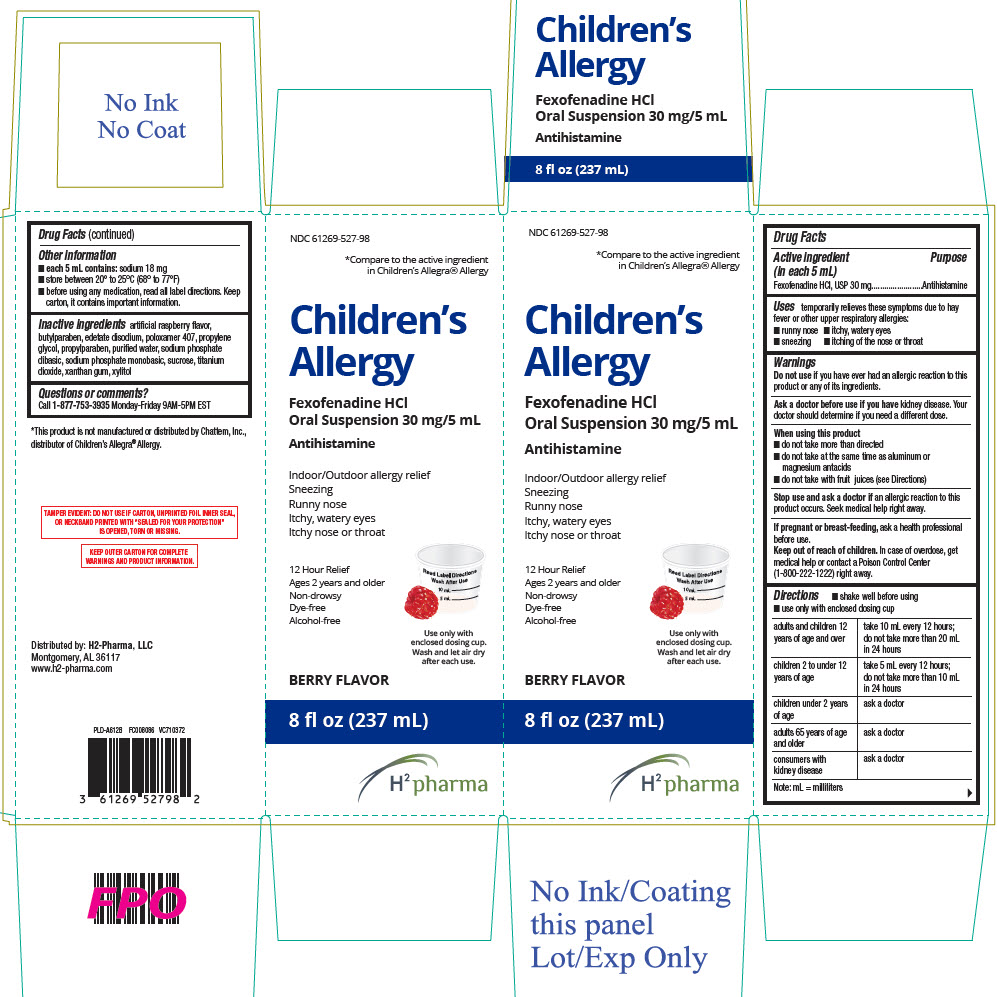
PRINCIPAL DISPLAY PANEL - 237 mL Bottle Carton
NDC 61269-527-98
*Compare to the active ingredient
in Children's Allegra® AllergyChildren's
AllergyFexofenadine HCl
Oral Suspension 30 mg/5 mLAntihistamine
Indoor/Outdoor allergy relief
Sneezing
Runny nose
Itchy, watery eyes
Itchy nose or throat12 Hour Relief
Ages 2 years and older
Non-drowsy
Dye-free
Alcohol-freeUse only with
enclosed dosing cup.
Wash and let air dry
after each use.BERRY FLAVOR
8 fl oz (237 mL)
H2 pharma
-
INGREDIENTS AND APPEARANCE
CHILDRENS ALLERGY
fexofenadine hydrochloride suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61269-527 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 30 mg in 5 mL Inactive Ingredients Ingredient Name Strength BUTYLPARABEN (UNII: 3QPI1U3FV8) EDETATE DISODIUM (UNII: 7FLD91C86K) POLOXAMER 407 (UNII: TUF2IVW3M2) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) SODIUM PHOSPHATE, MONOBASIC, ANHYDROUS (UNII: KH7I04HPUU) SUCROSE (UNII: C151H8M554) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) XANTHAN GUM (UNII: TTV12P4NEE) XYLITOL (UNII: VCQ006KQ1E) Product Characteristics Color Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61269-527-94 1 in 1 CARTON 07/18/2022 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:61269-527-98 1 in 1 CARTON 07/18/2022 2 237 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA203330 07/18/2022 Labeler - H2-Pharma, LLC (028473634)