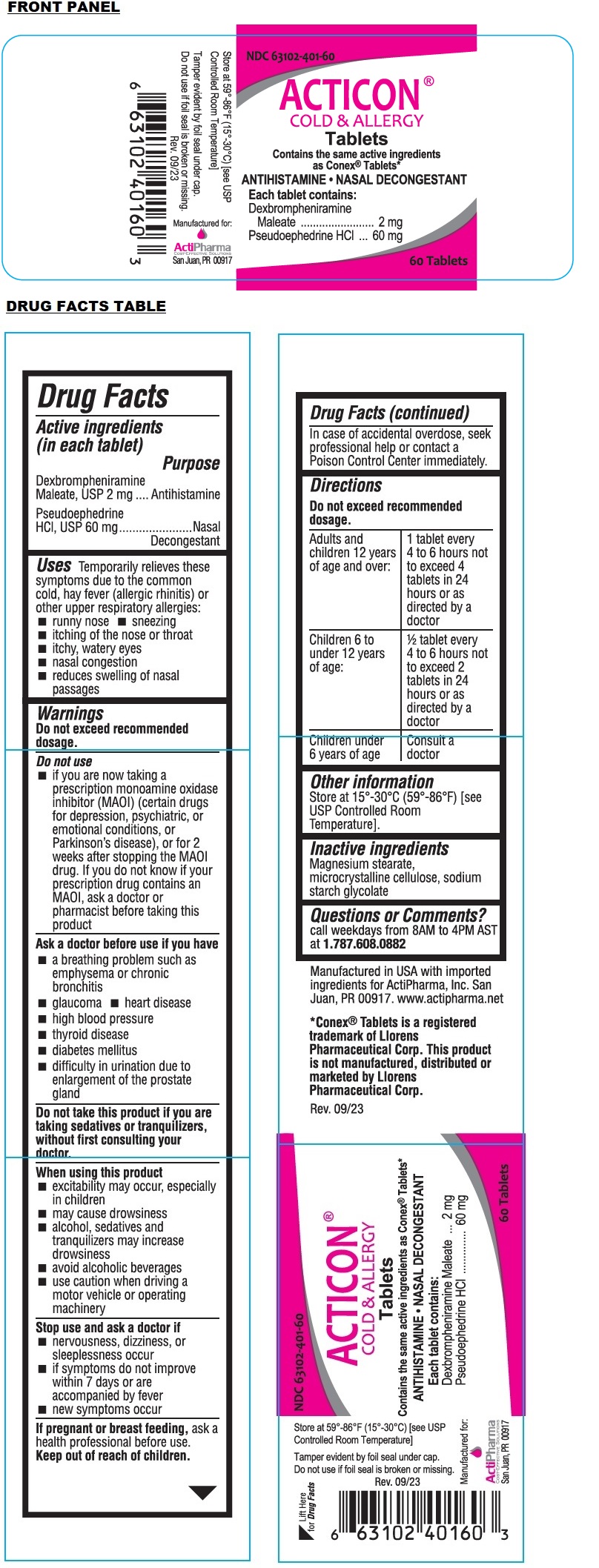
Label: ACTICON- dexbrompheniramine maleate, pseudoephedrine hydrochloride tablet
- NDC Code(s): 63102-401-60
- Packager: Actipharma, Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
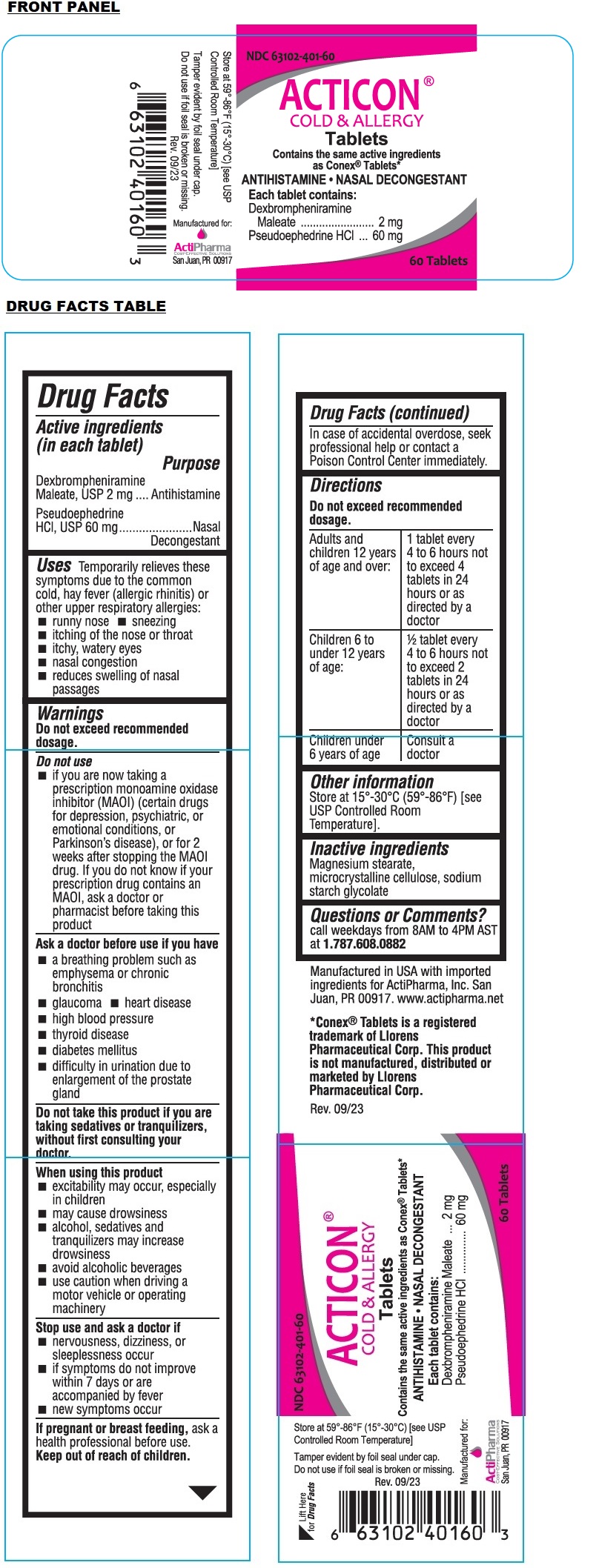
- Active ingredients (in each tablet)
- Purpose
- Uses
-
Warnings
Do not exceed recommended dosage.
Do not use
• if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this productAsk a doctor before use if you have
• a breathing problem such as emphysema or chronic bronchitis
• glaucoma • heart disease
• high blood pressure
• thyroid disease
• diabetes mellitus
• difficulty in urination due to enlargement of the prostate glandDo not take this product if you are taking sedatives or tranquilizers, without first consulting your doctor.
When using this product
• excitability may occur, especially in children
• may cause drowsiness
• alcohol, sedatives and tranquilizers may increase drowsiness
• avoid alcoholic beverages
• use caution when driving a motor vehicle or operating machineryStop use and ask a doctor if
• nervousness, dizziness, or sleeplessness occur
• if symptoms do not improve within 7 days or are accompanied by fever
• new symptoms occurIf pregnant or breast feeding, ask a health professional before use.
-
Directions
Do not exceed recommended dosage.
Adults and children 12 years of age and over: 1 tablet every 4 to 6 hours not to exceed 4 tablets in 24 hours or as directed by a doctor Children 6 to under 12 years of age: 1/2 tablet every 4 to 6 hours not to exceed 2 tablets in 24 hours or as directed by a doctor Children under
6 years of ageConsult a doctor - Other information
- Inactive ingredients
- Questions or Comments?
-
SPL UNCLASSIFIED SECTION
Contains the same active ingredients as Conex® Tablets*
Tamper evident by foil seal under cap.
Do not use if foil seal is broken or missing.Manufactured in USA with imported ingredients for ActiPharma, Inc. San Juan, PR 00917. www.actipharma.net
*Conex® Tablets is a registered trademark of Llorens Pharmaceutical Corp. This product is not manufactured, distributed or marketed by Llorens Pharmaceutical Corp.
- Packaging
-
INGREDIENTS AND APPEARANCE
ACTICON
dexbrompheniramine maleate, pseudoephedrine hydrochloride tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63102-401 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXBROMPHENIRAMINE MALEATE (UNII: BPA9UT29BS) (DEXBROMPHENIRAMINE - UNII:75T64B71RP) DEXBROMPHENIRAMINE MALEATE 2 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) Product Characteristics Color white Score 2 pieces Shape OVAL Size 13mm Flavor Imprint Code A401 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63102-401-60 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/13/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 08/13/2015 Labeler - Actipharma, Inc (079340948)