Label: WHITUBEN- acetaminophen, dextromethorphan hydrobromide hydrate, guaifenesin, pseudoephedrine hydrochloride, triprolidine hydrochloride hydrate capsule
-
Contains inactivated NDC Code(s)
NDC Code(s): 72689-0037-1 - Packager: OASIS TRADING
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 18, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
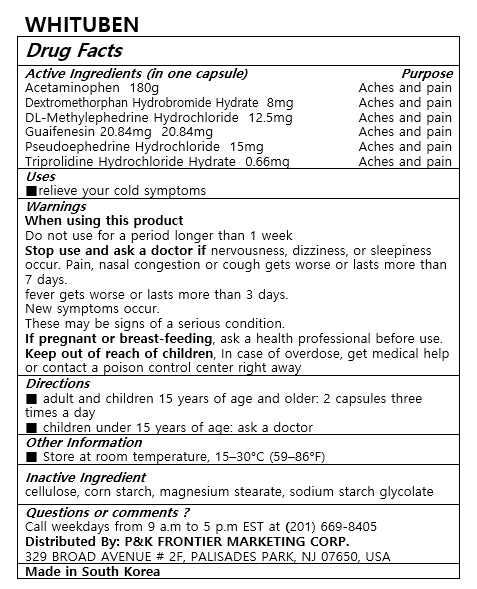
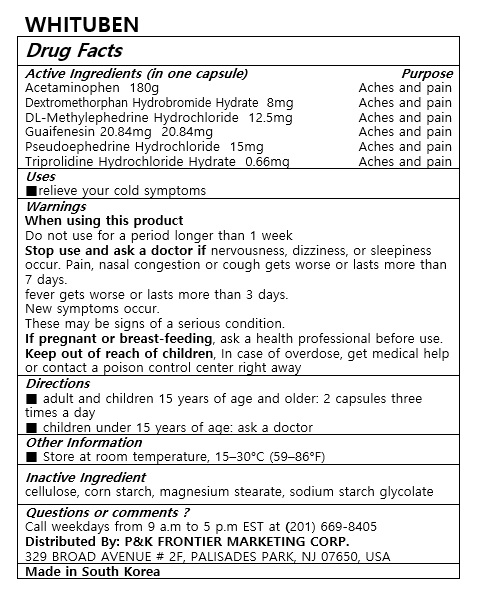
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
Warnings
When using this product
Do not use for a period longer than 1 weeks
Stop use and ask a doctor if nervousness, dizziness, or sleepness occur. Pain, nasal congestion or cough gets worse or lasts more than 7 days.
fever gets worse or lasts more than 3 days
New symptoms occur
These may be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children, In case of overdose, get medical help or contact a poison control center right away - INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WHITUBEN
acetaminophen, dextromethorphan hydrobromide hydrate, guaifenesin, pseudoephedrine hydrochloride, triprolidine hydrochloride hydrate capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72689-0037 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 180 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 20.84 mg PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 15 mg METHYLEPHEDRINE HYDROCHLORIDE, (+/-)- (UNII: 99214P83XM) (METHYLEPHEDRINE, (+/-)- - UNII:SHS9PGQ2LS) METHYLEPHEDRINE HYDROCHLORIDE, (+/-)- 12.5 mg TRIPROLIDINE HYDROCHLORIDE (UNII: YAN7R5L890) (TRIPROLIDINE - UNII:2L8T9S52QM) TRIPROLIDINE HYDROCHLORIDE 0.66 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 8 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color red Score no score Shape OVAL Size 14mm Flavor Imprint Code TWLQQ Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72689-0037-1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 11/22/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/22/2018 Labeler - OASIS TRADING (689991468) Registrant - OASIS TRADING (689991468) Establishment Name Address ID/FEI Business Operations OASIS TRADING 689991468 manufacture(72689-0037) , label(72689-0037)