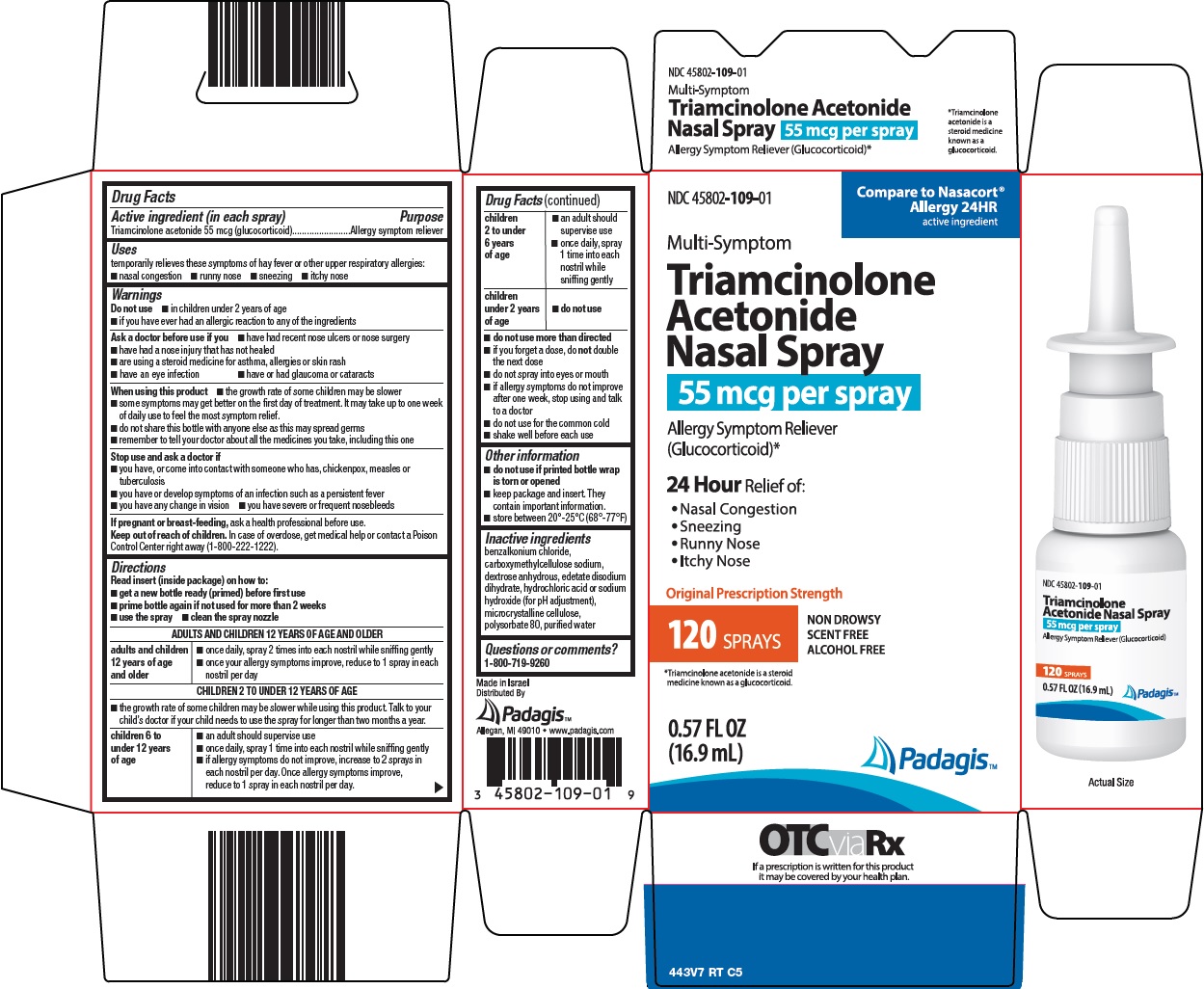
Label: TRIAMCINOLONE ACETONIDE NASAL- triamcinolone acetonide spray, metered
- NDC Code(s): 45802-109-01
- Packager: Padagis Israel Pharmaceuticals Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each spray)
- Purpose
- Uses
-
Warnings
Do not use
- •
- in children under 2 years of age
- •
- if you have ever had an allergic reaction to any of the ingredients
Ask a doctor before use if you
- •
- have had recent nose ulcers or nose surgery
- •
- have had a nose injury that has not healed
- •
- are using a steroid medicine for asthma, allergies or skin rash
- •
- have an eye infection
- •
- have or had glaucoma or cataracts
When using this product
- •
- the growth rate of some children may be slower
- •
- some symptoms may get better on the first day of treatment. It may take up to one week of daily use to feel the most symptom relief.
- •
- do not share this bottle with anyone else as this may spread germs
- •
- remember to tell your doctor about all the medicines you take, including this one
-
Directions
Read insert (inside package) on how to:
- •
- get a new bottle ready (primed) before first use
- •
- prime bottle again if not used for more than 2 weeks
- •
- use the spray
- •
- clean the spray nozzle
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER
adults and children
12 years of age
and older
- •
- once daily, spray 2 times into each nostril while sniffing gently
- •
- once your allergy symptoms improve, reduce to 1 spray in each nostril per day
CHILDREN 2 TO UNDER 12 YEARS OF AGE
- •
- the growth rate of some children may be slower while using this product. Talk to your child’s doctor if your child needs to use the spray for longer than two months a year.
children 6 to under
12 years of age
- •
- an adult should supervise use
- •
- once daily, spray 1 time into each nostril while sniffing gently
- •
- if allergy symptoms do not improve, increase to 2 sprays in each nostril per day. Once allergy symptoms improve, reduce to 1 spray in each nostril per day.
children 2 to under 6 years of age
- •
- an adult should supervise use
- •
- once daily, spray 1 time into each nostril while sniffing gently
children under 2 years of age
- •
- do not use
- •
- do not use more than directed
- •
- if you forget a dose, do not double the next dose
- •
- do not spray into eyes or mouth
- •
- if allergy symptoms do not improve after one week, stop using and talk to a doctor
- •
- do not use for the common cold
- •
- shake well before each use
- Other information
- Inactive ingredients
- Questions or comments?
-
Consumer information
Triamcinolone Acetonide Nasal Spray 55 mcg per spray
IMPORTANT INFORMATION
- •
- Read both sides of this insert for complete instructions on how to get a bottle ready (primed), how to use the spray bottle and how to clean the spray nozzle.
- •
- Keep this insert as it contains important information.
STEPS TO GET A NEW BOTTLE READY FOR USE (PRIMED)
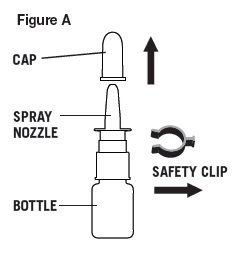
1. Before first use, a new bottle must be primed.
- a.
- Remove cap and safety clip (Figure A).
- b.
- Shake bottle.
- c.
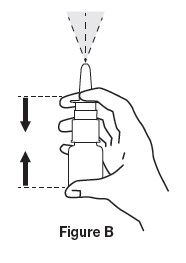
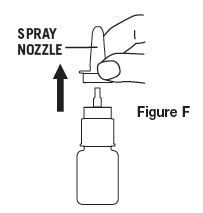
- Press and release spray nozzle until a fine mist is produced (as shown in Figure B). This may take several times. Take care not to spray in face.
PRIME BOTTLE AGAIN IF NOT USED FOR MORE THAN 2 WEEKS
- •
- Repeat steps for priming a bottle (see above).
USE INSTRUCTIONS
- 1.
- Blow nose gently to clear nostrils.
- 2.
- Remove cap and safety clip, then shake bottle.
- 3.
- Hold bottle with thumb under bottle and spray nozzle between fingers (as shown in Figure B).
- 4.
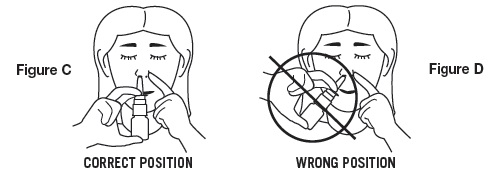
- Press against the outside of your nose with your finger to close off one nostril (as shown in Figure C).
- 5.
- Place the tip of the spray nozzle into the other nostril. The spray nozzle should not reach far into the nose. Aim nozzle toward back of nose (as shown in Figure C).
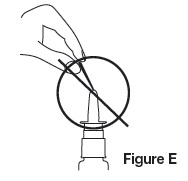
DO NOT spray toward nasal septum (the wall between the 2 nostrils) (as shown in Figure D).
- 6. While sniffing gently, spray into nostril.
IMPORTANT: For complete dosing instructions, See “Directions for use” on next side.
- 7. Repeat steps “4” through “6” for the other nostril.
- 8. After using the nasal spray, wipe nozzle with a tissue, replace safety clip, and replace cap by pressing it down over the spray nozzle.
NOTE: Avoid blowing nose for 15 minutes after use.
If nozzle does not spray properly, see cleaning instructions on next side.
(continued on side 2)
SIDE 1
DIRECTIONS FOR USE
- •
- BEFORE first use, you must get a new bottle ready (primed).
- •
- If bottle is not used for more than 2 weeks, prime bottle again.
Dosing
ADULTS AND CHILDREN 12 YEARS OF AGE AND OLDER
adults and children 12 years of age and older
- •
- once daily, spray 2 times into each nostril while sniffing gently
- •
- once your allergy symptoms improve, reduce to 1 spray in each nostril per day
CHILDREN 2 TO UNDER 12 YEARS OF AGE
- •
- the growth rate of some children may be slower while using this product. Talk to your child’s doctor if your child needs to use the spray for longer than two months a year.
children 6 to under 12 years of age
- •
- an adult should supervise use
- •
- once daily, spray 1 time into each nostril while sniffing gently
- •
- if allergy symptoms do not improve, increase to 2 sprays in each nostril per day. Once allergy symptoms improve, reduce to 1 spray in each nostril per day.
children 2 to under 6 years of age
- •
- an adult should supervise use
- •
- once daily, spray 1 time into each nostril while sniffing gently
children under 2 years of age
- •
- do not use
Other important information for use
- •
- Do not use more than directed.
- •
- If you forget a dose, do not double the next dose.
- •
- Do not spray into eyes or mouth.
- •
- If you get the spray in your eyes, rinse well with water.
- •
- If allergy symptoms do not improve after one week, stop using and talk to a doctor.
- •
- Do not share this bottle with anyone else as this may spread germs.
IF PUMP DOES NOT SPRAY PROPERLY, THE NOZZLE MAY BE BLOCKED
- 1.
- Never try to unblock nozzle with a pin or any other object (as shown in Figure E).
- 2.
- Clean the nozzle as shown below.
CLEANING INSTRUCTIONS
- 1.
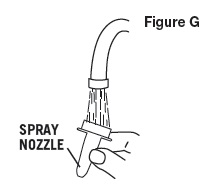
- Gently pull spray nozzle away from bottle (as shown in Figure F).
- 2.
- Rinse SPRAY NOZZLE ONLY under warm water (as shown in Figure G).
- 3.
- Shake or tap to remove excess water.
- 4.
- Re-attach spray nozzle to bottle.
- 5.
- Press and release spray nozzle until a fine spray is produced, taking care not to spray in face.
Triamcinolone Acetonide Nasal Spray pump is now ready to use.
Where can I get more information? 1-800-719-9260
Store between 20°-25°C (68°-77°F)
Made in Israel
Distributed By
Perrigo®
Allegan, MI 49010
SIDE 2
: 44300 00 J5
-
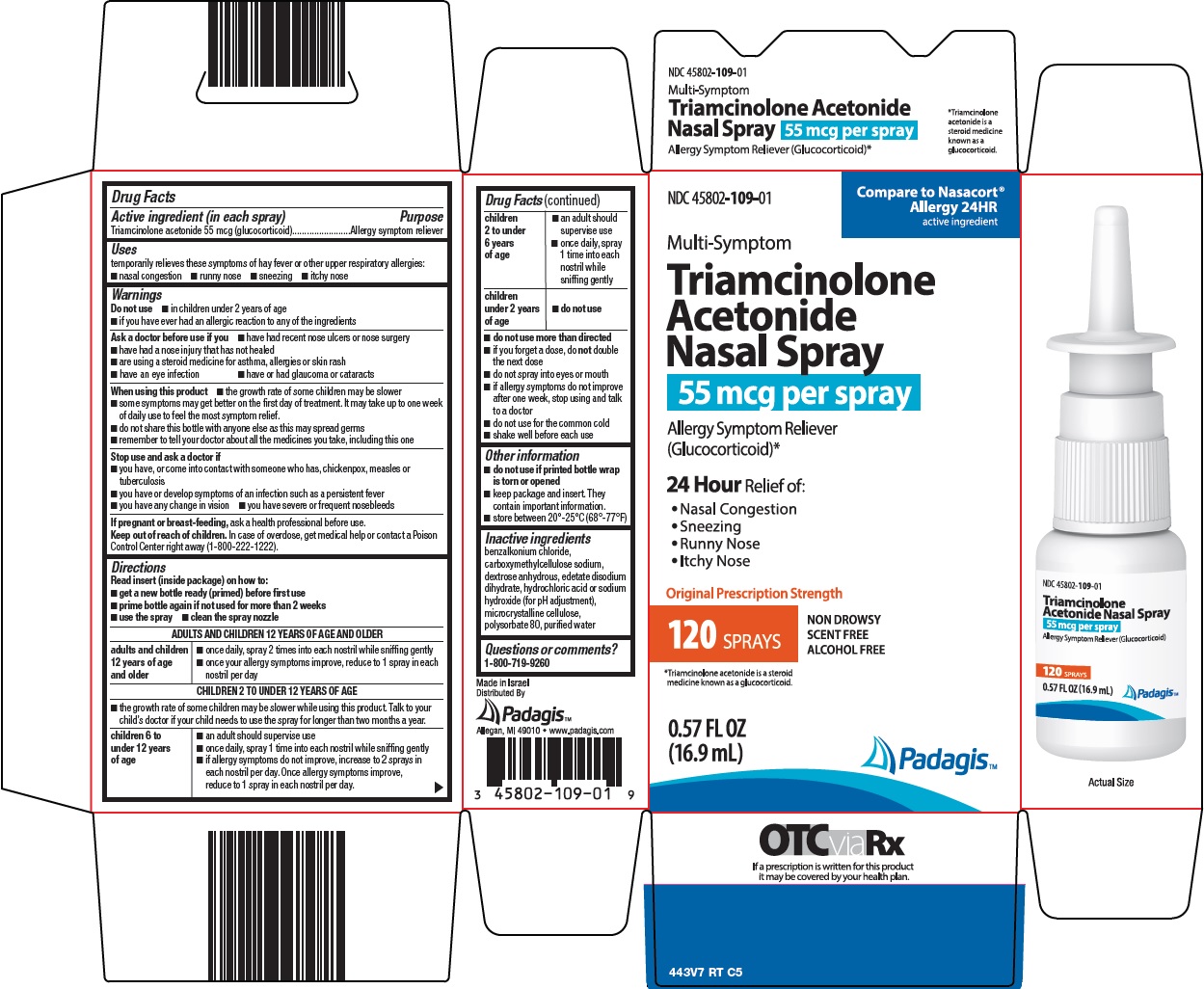
Package/Label Principal Display Panel
Compare to Nasacort® Allergy 24HR
active ingredient
Multi-Symptom Triamcinolone Acetonide Nasal Spray
55 mcg per spray
Allergy Symptom Reliever (Glucocorticoid)*
24 Hour Relief of:
• Nasal Congestion
• Sneezing
• Runny Nose
• Itchy Nose
Original Prescription Strength
NON DROWSY
SCENT FREE
ALCOHOL FREE
120 SPRAYS
*Triamcinolone acetonide is a steroid medicine known as a glucocorticoid.
0.57 FL OZ (16.9 mL)
Padagis™
-
INGREDIENTS AND APPEARANCE
TRIAMCINOLONE ACETONIDE NASAL
triamcinolone acetonide spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:45802-109 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRIAMCINOLONE ACETONIDE (UNII: F446C597KA) (TRIAMCINOLONE ACETONIDE - UNII:F446C597KA) TRIAMCINOLONE ACETONIDE 55 ug Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED (UNII: K679OBS311) ANHYDROUS DEXTROSE (UNII: 5SL0G7R0OK) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYSORBATE 80 (UNII: 6OZP39ZG8H) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (off white) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:45802-109-01 1 in 1 CARTON 03/28/2017 1 120 in 1 BOTTLE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078104 03/28/2017 Labeler - Padagis Israel Pharmaceuticals Ltd (600093611)