Label: CERTAIN DRI AM ROLL-ON- aluminum zirconium tetrachlorohydrex glycine liquid
- NDC Code(s): 69693-718-25
- Packager: Clarion Brands, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 22, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
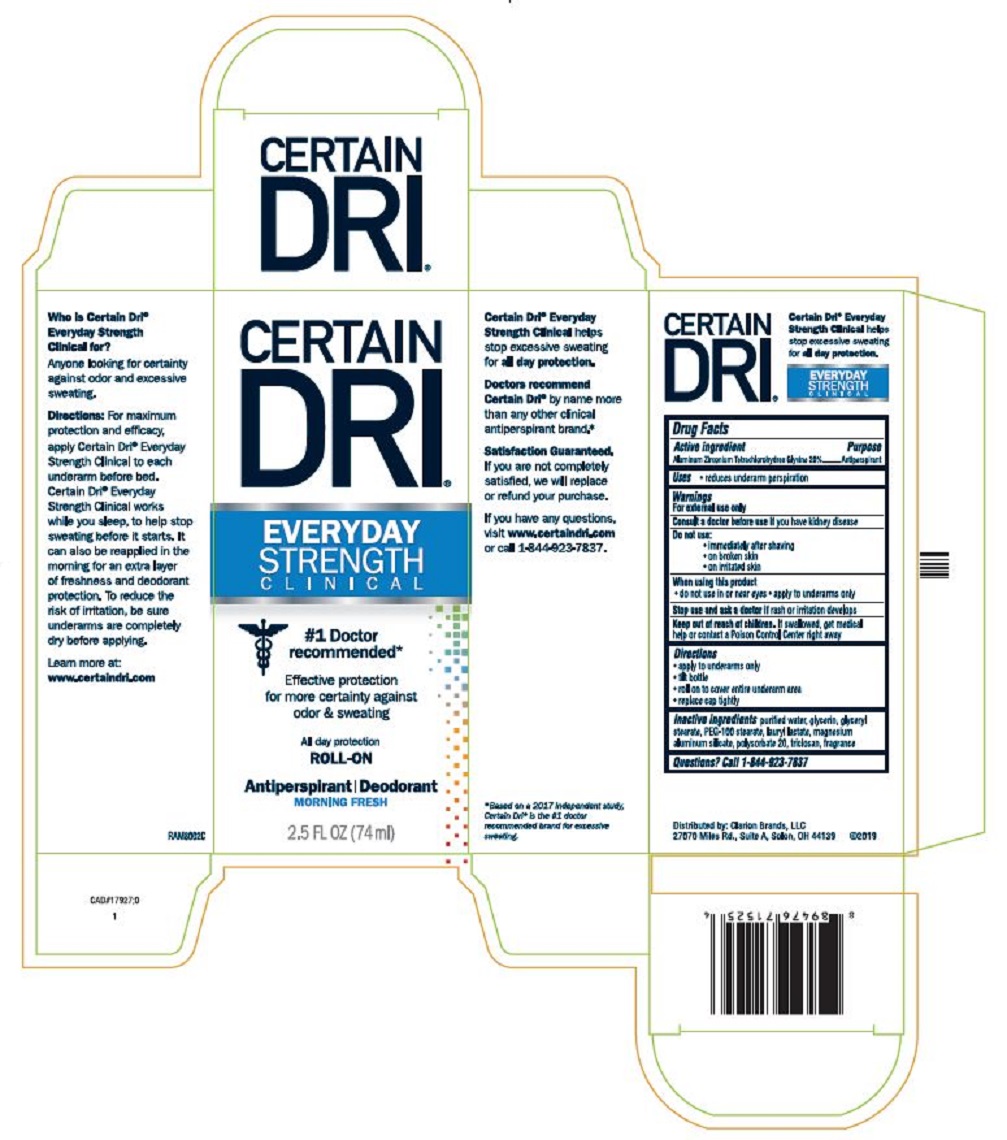
- ACTIVE INGREDIENT
- PURPOSE
- Uses
- Warnings
- ASK DOCTOR
- Do not use:
- When using this product
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Inactive ingredients
- QUESTIONS
-
PRINCIPAL DISPLAY PANEL
CERTAIN DRI®
EVERYDAY
STRENGTH
CLINICAL
#1 Doctor recommended*
Effective protection
for more certainty against
odor & sweating
All day protection
ROLL-ON
Antiperspirant | Deodorant
MORNING FRESH
2.5 FL OZ (74 ml)
Who is Certain Dri® Everyday Strength Clinical for?
Anyone looking for certainty against odor and excessive sweating.
Directions: For maximum protection and efficacy, apply Certain Dri® Everyday Strength Clinical to each underarm before bed. Certain Dri® Everyday Strength Clinical works while you sleep, to help stop sweating before it starts. It can also be reapplied in the morning for an extra layer of freshness and deodorant protection. To reduce the risk of irritation, be sure underarms are completely dry before applying.
Learn more at:
www.certaindri.com
RAM8002C
Certain Dri® Everyday Strength Clinical helps stop excessive sweating for all day protection.
Doctors recommend Certain Dri® by name more than any other clinical antiperspirant brand.*
Satisfaction Guaranteed.
If you are not completely satisfied, we will replace or refund your purchase.
If you have any questions, visit www.certaindri.com or call 1-844-923-7837.
*Based on a 2017 independent study, Certain Dri® is the #1 doctor recommended brand for excessive sweating.
Certain Dri® Everyday Strength Clinical helps stop excessive sweating for all day protection.
Distributed by: Clarion Brands, LLC
27070 Miles Rd., Suite A, Solon, OH 44139 ©2019
CERTAIN DRI®
EVERYDAY
STRENGTH
CLINICAL
#1 Doctor
recommended
Effective protection
for more certainty against
odor & sweating
All day protection
ROLL-ON
Antiperspirant | Deodorant
MORNING FRESH
2.5 FL OZ (74 ml)
Distributed by: Clarion Brands, LLC 27070 Miles Rd., Suite A, Solon, OH 44139 ©2019
RAM8004A
-
INGREDIENTS AND APPEARANCE
CERTAIN DRI AM ROLL-ON
aluminum zirconium tetrachlorohydrex glycine liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69693-718 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Aluminum Zirconium Tetrachlorohydrex Gly (UNII: 8O386558JE) (Aluminum Cation - UNII:3XHB1D032B) Aluminum Zirconium Tetrachlorohydrex Gly 20 g in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Glycerin (UNII: PDC6A3C0OX) Glyceryl Monostearate (UNII: 230OU9XXE4) Peg-100 Stearate (UNII: YD01N1999R) Magnesium Aluminum Silicate (UNII: 6M3P64V0NC) Polysorbate 20 (UNII: 7T1F30V5YH) Triclosan (UNII: 4NM5039Y5X) Lauryl Lactate (UNII: G5SU0BFK7O) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69693-718-25 1 in 1 CARTON 06/16/2015 1 74 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 06/16/2015 Labeler - Clarion Brands, LLC (079742703)