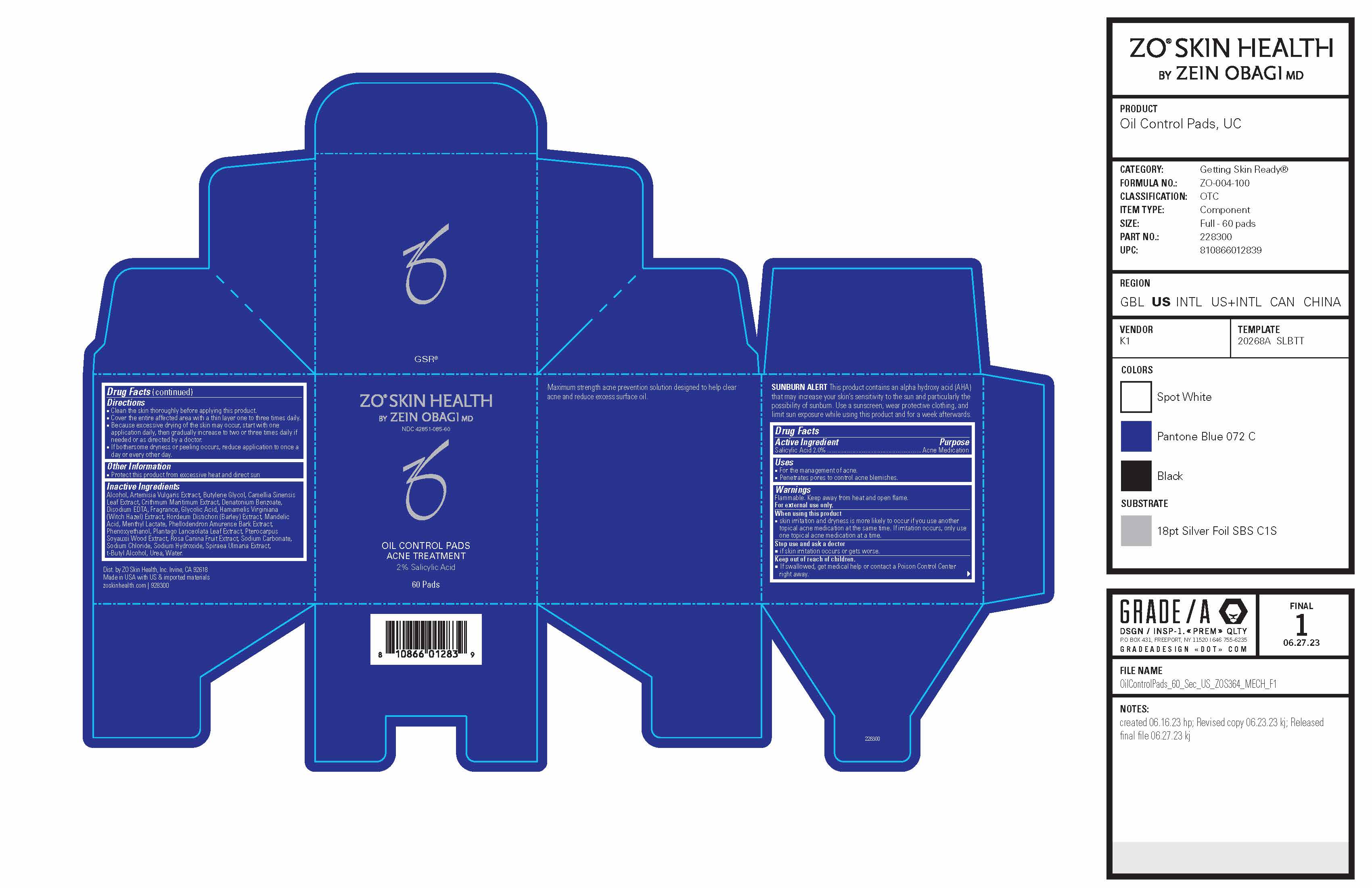
Label: ZO SKIN HEALTH OIL CONTROL PADS ACNE TREATMENT- salicylic acid patch
- NDC Code(s): 42851-085-30, 42851-085-60
- Packager: ZO Skin Health, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 1, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
- Clean the skin thoroughly before applying this product.
- Cover the entire affected area with a thin layer one to three times daily.
- Because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor.
- If bothersome dryness or peeling occurs, reduce application to once a day or every other day.
- Other information
-
Inactive ingredients
Alcohol, Artemisia Vulgaris Extract, Butylene Glycol, Camellia Sinensis Leaf Extract, Crithmum Maritimum Extract, Fragrance, Glycolic Acid, Hamamelis Virginiana (Witch Hazel) Extract, Hordeum Vulgare Seed Extract, Mandelic Acid, Menthyl Lactate, Phenoxyethanol, Phellodendron Amureuse Extract, Plantago Lanceolata Leaf Extract, Pterocarpus Soyauxii Wood Extract, Rosa Canina Fruit Extract, Sodium Hydroxide, Spiraea Ulmaria Extract, Urea, Water.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 60 Pad Jar Carton
-
INGREDIENTS AND APPEARANCE
ZO SKIN HEALTH OIL CONTROL PADS ACNE TREATMENT
salicylic acid patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:42851-085 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength UREA (UNII: 8W8T17847W) WATER (UNII: 059QF0KO0R) MANDELIC ACID (UNII: NH496X0UJX) PHELLODENDRON AMURENSE BARK (UNII: PBG27B754G) GLYCOLIC ACID (UNII: 0WT12SX38S) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) DENATONIUM BENZOATE (UNII: 4YK5Z54AT2) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) GREEN TEA LEAF (UNII: W2ZU1RY8B0) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CRITHMUM MARITIMUM (UNII: J7IHY79BKY) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CARBONATE (UNII: 45P3261C7T) HAMAMELIS VIRGINIANA TOP (UNII: UDA30A2JJY) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) PLANTAGO LANCEOLATA LEAF (UNII: 2YWL9J7EE8) PTEROCARPUS SOYAUXII WOOD (UNII: 0V6QB4C61P) ROSA CANINA FRUIT (UNII: 3TNW8D08V3) ALCOHOL (UNII: 3K9958V90M) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42851-085-60 1 in 1 CARTON 02/01/2024 1 60 in 1 JAR 1 0.8 mL in 1 PATCH; Type 0: Not a Combination Product 2 NDC:42851-085-30 1 in 1 CARTON 02/01/2024 2 30 in 1 JAR 2 0.8 mL in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 02/01/2024 Labeler - ZO Skin Health, Inc. (826468527)