Label: EEZYSUN SUNSCREEN SPF 50 WITH ALOE VERA, MARINE FRIENDLY- titanium dioxide,zinc oxide cream
- NDC Code(s): 70116-012-01
- Packager: BIO EARTH MANUFACTURING (PTY) LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
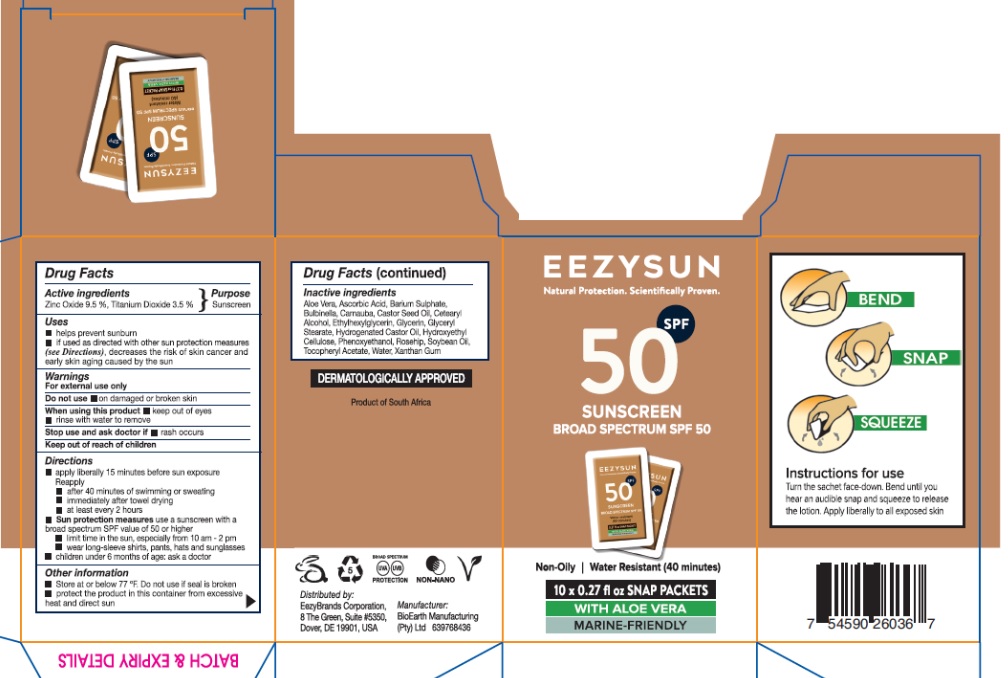
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
- Keep out of reach of children
-
Directions
- Apply liberally 15 minutes before sun exposure
Reapply
- after 40 minutes if swimming or sweating
- immediately after towel drying
- at least every two hours
Sun protection measuresuse a broad-spectrum sunscreen with an SPF value of 50 or higher
- limit time in the sun, especially from 10am - 2pm
- wear long-sleeve shirts, pants, hats, sunglasses
- Children under 6 months of age: ask a doctor
- Other Information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EEZYSUN SUNSCREEN SPF 50 WITH ALOE VERA, MARINE FRIENDLY
titanium dioxide,zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70116-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 3.5 g in 100 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 9.5 g in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF POLYSACCHARIDES (UNII: W21O437517) ASCORBIC ACID (UNII: PQ6CK8PD0R) BARIUM SULFATE (UNII: 25BB7EKE2E) BULBINE FRUTESCENS WHOLE (UNII: M2U1C7UW6Y) CARNAUBA WAX (UNII: R12CBM0EIZ) CASTOR OIL (UNII: D5340Y2I9G) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) PHENOXYETHANOL (UNII: HIE492ZZ3T) ROSA MOSCHATA OIL (UNII: J99W255AWF) SOYBEAN OIL (UNII: 241ATL177A) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70116-012-01 100 mL in 1 TUBE; Type 0: Not a Combination Product 03/30/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 03/30/2021 Labeler - BIO EARTH MANUFACTURING (PTY) LTD (639768436) Establishment Name Address ID/FEI Business Operations BIO EARTH MANUFACTURING (PTY) LTD 639768436 manufacture(70116-012)