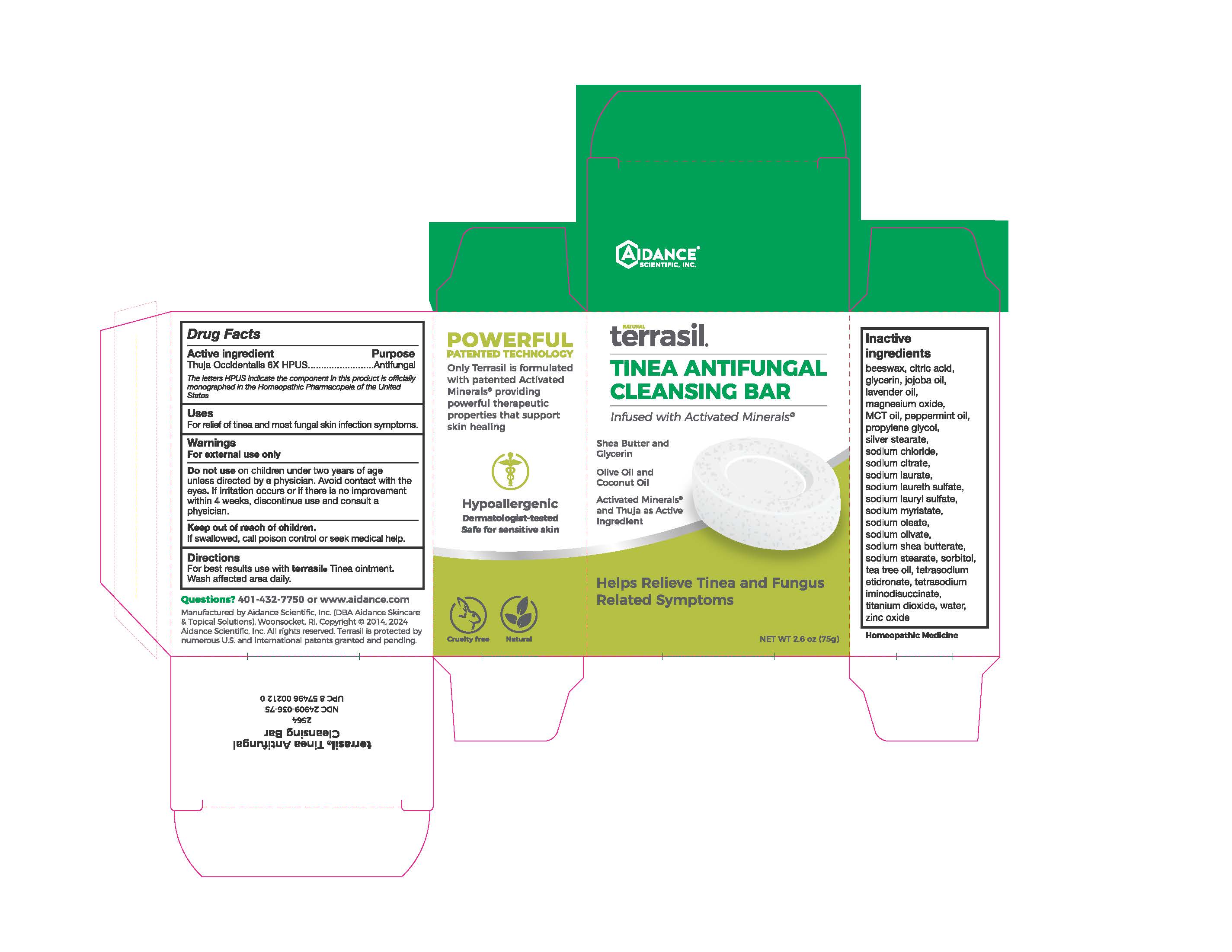
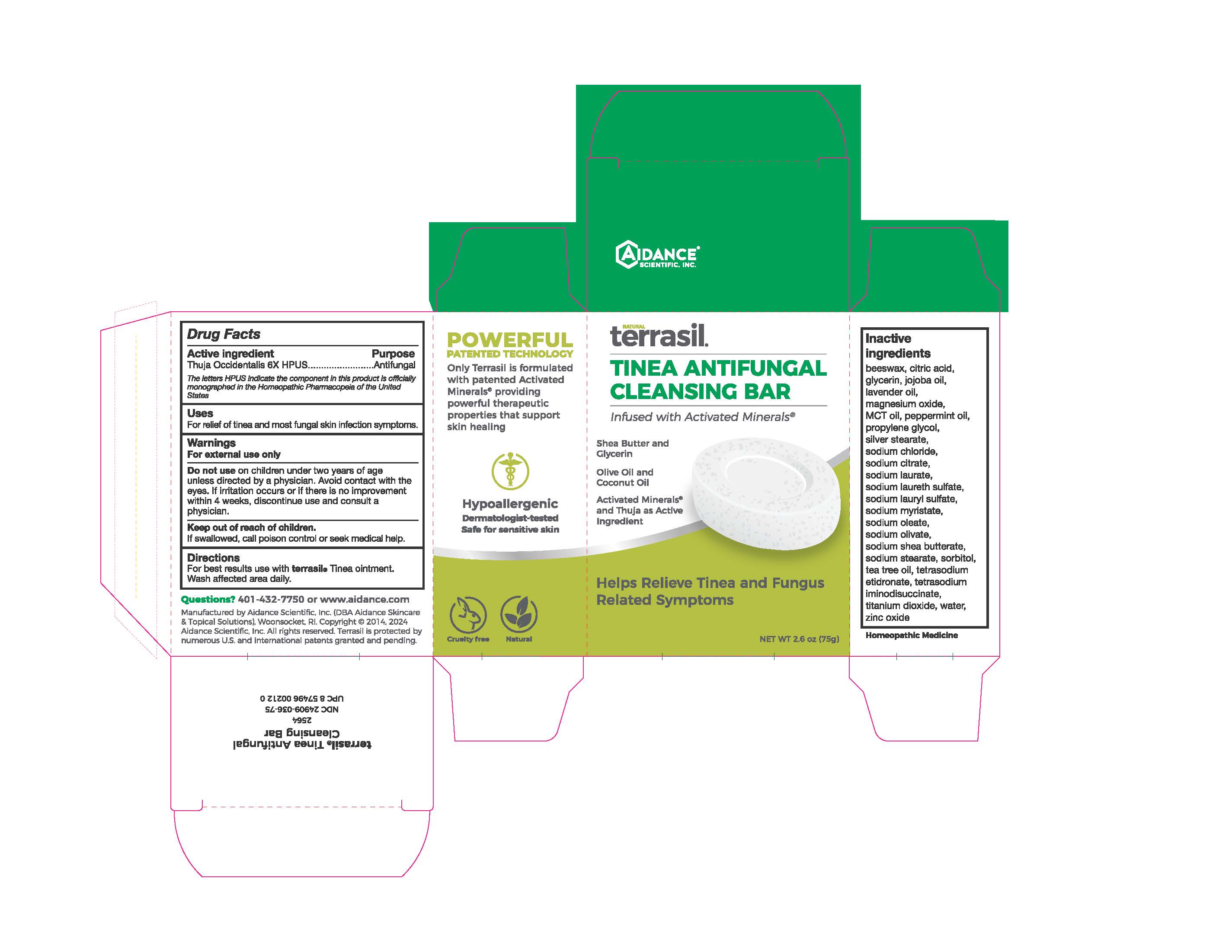
Label: TERRASIL TINEA ANTIFUNGAL CLEANSING BAR- thuja occidentalis whole soap
- NDC Code(s): 24909-036-75
- Packager: Aidance Scientific, Inc, DBA Aidance Skincare & Topical Solutions
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive Ingredients
beeswax, citric acid, glycerin, jojoba oil, lavender oil, magnesium oxide, peppermint oil, propylene glycol, silver stearate, sodium chloride, sodium citrate, sodium laurate, sodium laureth sulfate, sodium lauryl sulfate, sodium myristate, sodium oleate, sodium olivate, sodium shea butterate, sodium stearate, sorbitol, tea tree oil, tetrasodium etidronate, tetrasodium iminodisuccinate, titanium dioxide, water, zinc oxide
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TERRASIL TINEA ANTIFUNGAL CLEANSING BAR
thuja occidentalis whole soapProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24909-036 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength THUJA OCCIDENTALIS WHOLE (UNII: 5HBV6WCE3N) (THUJA OCCIDENTALIS WHOLE - UNII:5HBV6WCE3N) THUJA OCCIDENTALIS WHOLE 6 [hp_X] in 1 g Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHEA BUTTER (UNII: K49155WL9Y) YELLOW WAX (UNII: 2ZA36H0S2V) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) JOJOBA OIL (UNII: 724GKU717M) GLYCERIN (UNII: PDC6A3C0OX) LAVENDER OIL (UNII: ZBP1YXW0H8) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) PEPPERMINT OIL (UNII: AV092KU4JH) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM LAURATE (UNII: K146MR5EXO) SODIUM LAURETH SULFATE (UNII: BPV390UAP0) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SODIUM MYRISTATE (UNII: 06BLC4V0IV) SODIUM OLEATE (UNII: 399SL044HN) SODIUM OLIVATE (UNII: ND5Y5M6ZUT) SODIUM STEARATE (UNII: QU7E2XA9TG) SORBITOL (UNII: 506T60A25R) TEA TREE OIL (UNII: VIF565UC2G) ETIDRONATE TETRASODIUM (UNII: CZZ9T1T1X4) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) ZINC OXIDE (UNII: SOI2LOH54Z) SILVER STEARATE (UNII: 4H6PCL92ZN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) TETRASODIUM IMINODISUCCINATE (UNII: GYS41J2635) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24909-036-75 75 g in 1 BOX; Type 0: Not a Combination Product 09/01/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/01/2023 Labeler - Aidance Scientific, Inc, DBA Aidance Skincare & Topical Solutions (018950611) Establishment Name Address ID/FEI Business Operations Aidance Scientific, Inc, DBA Aidance Skincare & Topical Solutions 018950611 manufacture(24909-036)