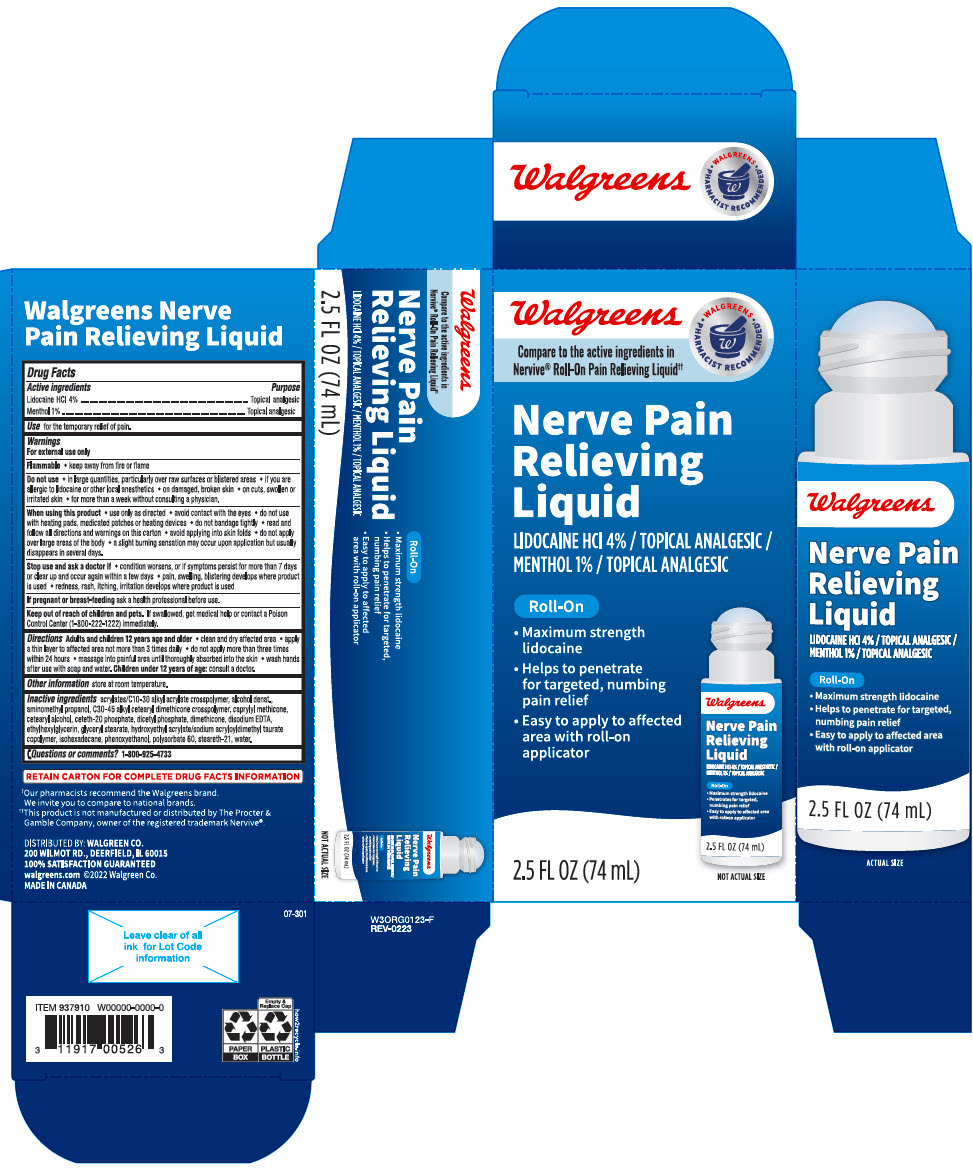
Label: WALGREENS NERVE PAIN RELIEVING TOPICAL ANALGESIC- lidocaine hydrochloride and menthol, unspecified form liquid
- NDC Code(s): 0363-3791-01
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 6, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- ACTIVE INGREDIENT
- Use
-
Warnings
For external use only
Do not use
- ⬥
- in large quantities, particularly over raw surfaces or blistered areas
- ⬥
- if you are allergic to lidocaine or other local anesthetics
- ⬥
- on damaged, broken skin
- ⬥
- on cuts, swollen or irritated skin
- ⬥
- for more than a week without consulting a physician.
When using this product
- ⬥
- use only as directed
- ⬥
- avoid contact with the eyes
- ⬥
- do not use with heating pads, medicated patches or heating devices
- ⬥
- do not bandage tightly
- ⬥
- read and follow all directions and warnings on this carton
- ⬥
- avoid applying into skin folds
- ⬥
- do not apply over large areas of the body
- ⬥
- a slight burning sensation may occur upon application but usually disappears in several days.
-
Directions
Adults and children 12 years of age and older
- ⬥
- clean and dry affected area
- ⬥
- apply a thin layer to affected area not more than 3 times daily
- ⬥
- do not apply more than three times within 24 hours
- ⬥
- massage into painful area until thoroughly absorbed into the skin
- ⬥
- wash hands after use with soap and water.
Children under 12 years of age: consult a doctor.
- Other information
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, alcohol denat., aminomethyl propanol, C30-45 alkyl cetearyl dimethicone crosspolymer, caprylyl methicone, cetearyl alcohol, ceteth-20 phosphate, dicetyl phosphate, dimethicone, disodium EDTA, ethylhexylglycerin, glyceryl stearate, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer, isohexadecane, phenoxyethanol, polysorbate 60, steareth-21, water.
- Questions or comments?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 74 mL Cylinder Carton
Walgreens
Compare to the active ingredients in
Nervive® Roll-On Pain Relieving Liquid††WALGREENS
• PHARMACIST RECOMMENDED† •Nerve Pain
Relieving
LiquidLIDOCAINE HCl 4% / TOPICAL ANALGESIC /
MENTHOL 1% / TOPICAL ANALGESICRoll-On
- Maximum strength
lidocaine - Helps to penetrate
for targeted, numbing
pain relief - Easy to apply to affected
area with roll-on
applicator
NOT ACTUAL SIZE
2.5 FL OZ (74 mL)
- Maximum strength
-
INGREDIENTS AND APPEARANCE
WALGREENS NERVE PAIN RELIEVING TOPICAL ANALGESIC
lidocaine hydrochloride and menthol, unspecified form liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-3791 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Lidocaine Hydrochloride (UNII: V13007Z41A) (Lidocaine - UNII:98PI200987) Lidocaine Hydrochloride Anhydrous 40 mg in 1 mL Menthol, Unspecified Form (UNII: L7T10EIP3A) (Menthol, Unspecified Form - UNII:L7T10EIP3A) Menthol, Unspecified Form 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength Carbomer Interpolymer Type A (Allyl Sucrose Crosslinked) (UNII: 59TL3WG5CO) Alcohol (UNII: 3K9958V90M) Aminomethylpropanol (UNII: LU49E6626Q) C30-45 Alkyl Cetearyl Dimethicone Crosspolymer (UNII: 4ZK9VP326R) Caprylyl Trisiloxane (UNII: Q95M2P1KJL) Cetostearyl Alcohol (UNII: 2DMT128M1S) Ceteth-20 Phosphate (UNII: 921FTA1500) Dihexadecyl Phosphate (UNII: 2V6E5WN99N) Dimethicone (UNII: 92RU3N3Y1O) Edetate Disodium Anhydrous (UNII: 8NLQ36F6MM) Ethylhexylglycerin (UNII: 147D247K3P) Glyceryl Monostearate (UNII: 230OU9XXE4) Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) Isohexadecane (UNII: 918X1OUF1E) Phenoxyethanol (UNII: HIE492ZZ3T) Polysorbate 60 (UNII: CAL22UVI4M) Steareth-21 (UNII: 53J3F32P58) Water (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-3791-01 1 in 1 CARTON 02/21/2023 1 74 mL in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part348 02/21/2023 Labeler - Walgreen Company (008965063) Registrant - Garcoa, Inc. (036464697) Establishment Name Address ID/FEI Business Operations Sigan Industries INC. 255106239 MANUFACTURE(0363-3791)