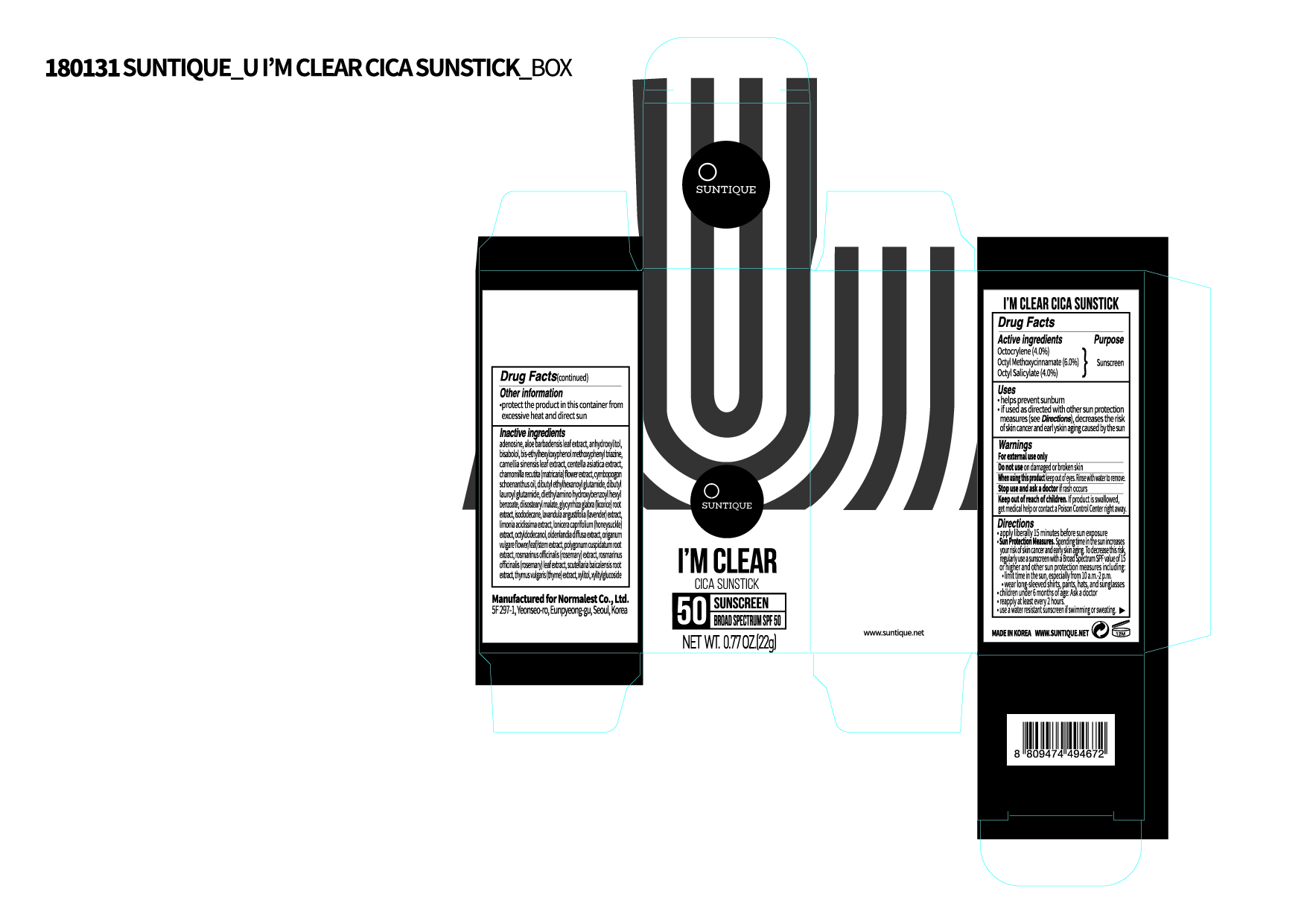
Label: IM CLEAR CICA SUNSTICK- octyl methoxycinnamate, octyl salicylate, octocrylene lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 72284-0002-1 - Packager: Normalest Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 5, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
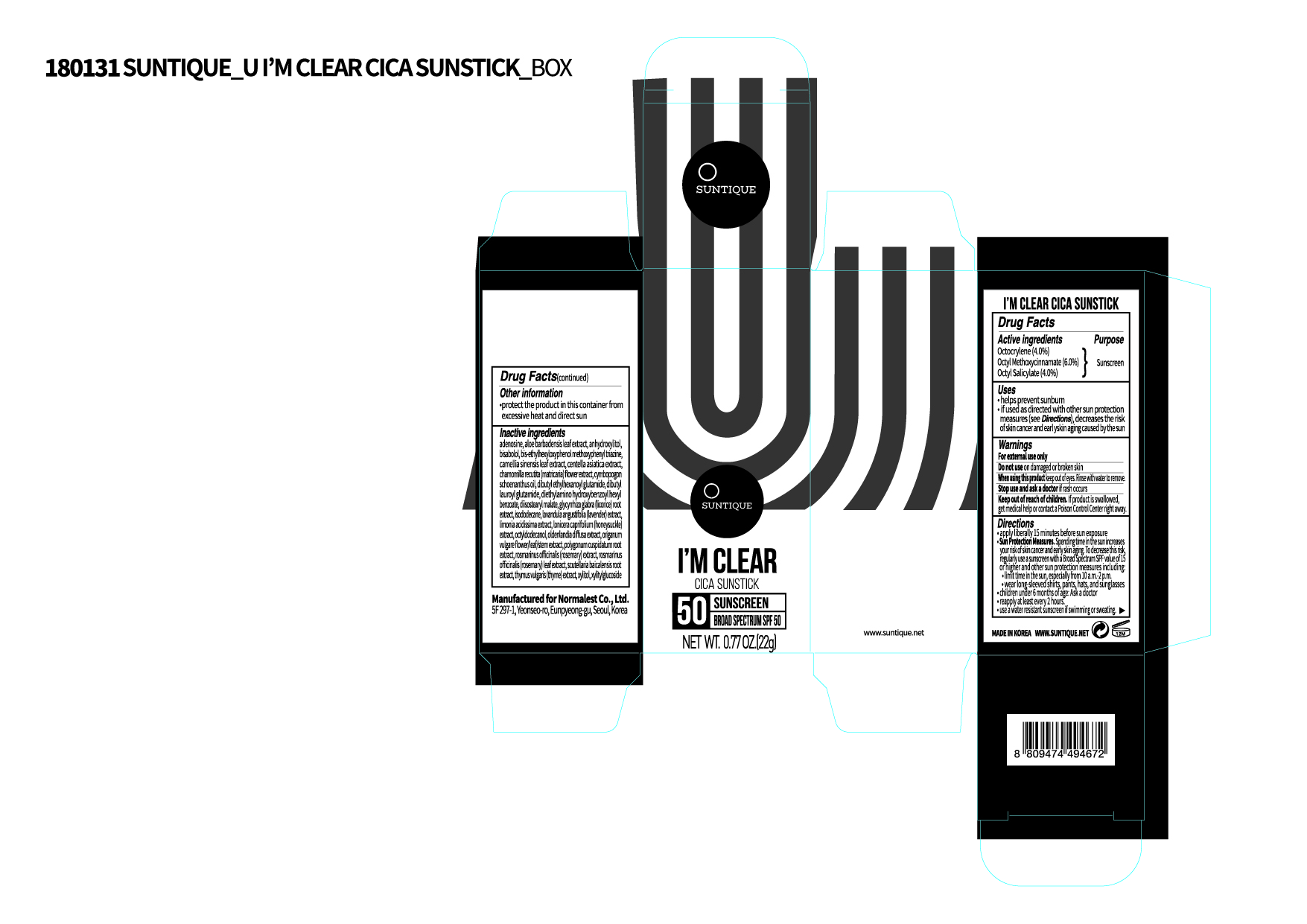
Inactive ingredients
Octyldodecanol, Isododecane, Diisostearyl Malate, Dibutyl Lauroyl Glutamide, Diethylamino Hydroxybenzoyl Hexyl Benzoate, Dibutyl Ethylhexanoyl Glutamide, Bis-Ethylhexyloxyphenol Methoxyphenyl Triazine, (-)-alpha-bisabolol, Cymbopogon Schoenanthus Oil, Adenosine, Aloe Barbadensis Leaf Extract, Xylitylglucoside, Anhydroxylitol, Xylitol, Limonia Acidissima Extract, Bambusa Vulgaris Leaf Extract, Oldenlandia Diffusa Extract, Lonicera Caprifolium (Honeysuckle) Extract, Lavandula Angustifolia (Lavender) Extract, Rosmarinus Officinalis (Rosemary) Extract, Origanum Vulgare Flower/Leaf/Stem Extract, Thymus Vulgaris (Thyme) Extract, Centella Asiatica Extract, Chamomilla Recutita (Matricaria) Flower Extract, Glycyrrhiza Glabra (Licorice) Root Extract, Camellia Sinensis Leaf Extract, Polygonum Cuspidatum Root Extract, Rosmarinus Officinalis (Rosemary) Leaf Extract, Scutellaria Baicalensis Root Extract
-
INGREDIENTS AND APPEARANCE
IM CLEAR CICA SUNSTICK
octyl methoxycinnamate, octyl salicylate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72284-0002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.88 g in 22 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 0.88 g in 22 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 1.32 g in 22 g Inactive Ingredients Ingredient Name Strength DIBUTYL LAUROYL GLUTAMIDE (UNII: 3V7K3IA58X) DIETHYLAMINO HYDROXYBENZOYL HEXYL BENZOATE (UNII: ANQ870JD20) BEMOTRIZINOL (UNII: PWZ1720CBH) ALOE VERA LEAF (UNII: ZY81Z83H0X) XYLITOL (UNII: VCQ006KQ1E) LIMONIA ACIDISSIMA WOOD (UNII: 04X926XD7Z) BAMBUSA VULGARIS LEAF (UNII: EMY54R518C) LAVANDULA ANGUSTIFOLIA WHOLE (UNII: 51217XIL5L) MATRICARIA CHAMOMILLA FLOWERING TOP (UNII: 3VNC7T6Z02) LONICERA CAPRIFOLIUM WHOLE (UNII: 4F67A99YTF) ORIGANUM VULGARE SUBSP. HIRTUM WHOLE (UNII: 38SNL0F81Z) THYMUS VULGARIS WHOLE (UNII: 8L72OKJ7II) GREEN TEA LEAF (UNII: W2ZU1RY8B0) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) ROSMARINUS OFFICINALIS WHOLE (UNII: EA3289138M) CENTELLA ASIATICA (UNII: 7M867G6T1U) POLYGONUM CUSPIDATUM ROOT (UNII: 7TRV45YZF7) CYMBOPOGON SCHOENANTHUS OIL (UNII: XE7K568ILO) ADENOSINE (UNII: K72T3FS567) XYLITYLGLUCOSIDE (UNII: O0IEZ166FB) ISODODECANE (UNII: A8289P68Y2) DIISOSTEARYL MALATE (UNII: QBS8A3XZGQ) DIBUTYL ETHYLHEXANOYL GLUTAMIDE (UNII: 0IAF2L30VS) OCTYLDODECANOL (UNII: 461N1O614Y) LEVOMENOL (UNII: 24WE03BX2T) ANHYDROXYLITOL (UNII: 8XWR7NN42F) OLDENLANDIA DIFFUSA (UNII: 291PPU5K9I) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) Product Characteristics Color yellow Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72284-0002-1 1 in 1 BOX 03/06/2019 1 22 g in 1 CASE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/06/2019 Labeler - Normalest Co., Ltd. (694812877) Registrant - Normalest Co., Ltd. (694812877) Establishment Name Address ID/FEI Business Operations Normalest Co., Ltd. 694812877 manufacture(72284-0002)