Label: K PHOS NEUTRAL- sodium phosphate, dibasic, anhydrous, potassium phosphate, monobasic, and sodium phosphate, monobasic, monohydrate tablet, coated
- NDC Code(s): 0486-1125-01, 0486-1125-05
- Packager: Beach Products, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated February 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Each tablet contains 852 mg dibasic sodium phosphate anhydrous, 155 mg monobasic potassium phosphate, and the equivalent of 130 mg monobasic sodium phosphate monohydrate. Each tablet yields approximately 250 mg of phosphorus, 298 mg of sodium (13.0 mEq) and 45 mg of potassium (1.1 mEq). The components of K-PHOS® NEUTRAL have the following chemical names and molecular formulae:
Dibasic Sodium Phosphate, Anhydrous
Molecular Formula: Na2HPO4, Molecular Weight: 141.96.Monobasic Potassium Phosphate
Molecular Formula: KH2PO4, Molecular Weight: 136.09.Monobasic Sodium Phosphate, Monohydrate
Molecular Formula:NaH2PO4.H2O, Molecular Weight: 137.98.Inactive Ingredients: Lactose monohydrate, povidone, white coating (hydroxypropyl methylcellulose, titanium dioxide, maltodextrin, triacetin, glycerol triacetate, polyethylene glycol, sodium citrate, and stearic acid), sodium starch glycolate, and magnesium stearate.
-
CLINICAL PHARMACOLOGY
Phosphorus has a number of important functions in the biochemistry of the body. The bulk of the body's phosphorus is located in the bones, where it plays a key role in osteoblastic and osteoclastic activities. Enzymatically catalyzed phosphate-transfer reactions are numerous and vital in the metabolism of carbohydrate, lipid and protein, and a proper concentration of the anion is of primary importance in assuring an orderly biochemical sequence. In addition, phosphorus plays an important role in modifying steady-state tissue concentrations of calcium. Phosphate ions are important buffers of the intracellular fluid, and also play a primary role in the renal excretion of hydrogen ion.
Oral administration of inorganic phosphates increases serum phosphate levels. Phosphates lower urinary calcium levels in idiopathic hypercalciuria.
In general, in adults, about two thirds of the ingested phosphate is absorbed from the bowel, most of which is rapidly excreted into the urine.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
This product contains potassium and sodium and should be used with caution if regulation of these elements is desired. Occasionally, some individuals may experience a mild laxative effect during the first few days of phosphate therapy. If laxation persists to an unpleasant degree, reduce the daily dosage until this effect subsides or, if necessary, discontinue the use of this product.
Caution should be exercised when prescribing this product in the following conditions: Cardiac disease (particularly in digitalized patients); severe adrenal insufficiency (Addison's disease); acute dehydration; severe renal insufficiency; renal function impairment or chronic renal disease; extensive tissue breakdown (such as severe burns); myotonia congenita; cardiac failure; cirrhosis of the liver or severe hepatic disease; peripheral or pulmonary edema; hypernatremia; hypertension; toxemia of pregnancy; hypoparathyroidism; and acute pancreatitis. Rickets may benefit from phosphate therapy, but caution should be exercised. High serum phosphate levels may increase the incidence of extra-skeletal calcification.
Information for Patients
Patients with kidney stones may pass old stones when phosphate therapy is started and should be warned of this possibility. Patients should be advised to avoid the use of antacids containing aluminum, magnesium, or calcium which may prevent the absorption of phosphate.
Laboratory Tests
Careful monitoring of renal function and serum calcium, phosphorus, potassium and sodium may be required at periodic intervals during phosphate therapy. Other tests may be warranted in some patients, depending on conditions.
Drug Interactions
The use of antacids containing magnesium, aluminum, or calcium in conjunction with phosphate preparations may bind the phosphate and prevent its absorption. Concurrent use of antihypertensives, especially diazoxide, guanethidine, hydralazine, methyldopa, or rauwolfia alkaloid; or corticosteroids, especially mineralocorticoids or corticotropin, with sodium phosphate may result in hypernatremia. Calciumcontaining preparations and/or Vitamin D may antagonize the effects of phosphates in the treatment of hypercalcemia. Potassium-containing medications or potassium-sparingdiuretics may cause hyperkalemia. Patients should have serum potassium level determinations at periodic intervals.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term or reproduction studies in animals or humans have been performed with K-PHOS ® NEUTRAL to evaluate its carcinogenic, mutagenic, or impairment of fertility potential.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Animal reproduction studies have not been conducted with K-PHOS® NEUTRAL. It is also not known whether this product can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. This product should be given to a pregnant woman only if clearly needed.
-
ADVERSE REACTIONS
Gastrointestinal upset (diarrhea, nausea, stomach pain and vomiting) may occur with phosphate therapy. Also, bone and joint pain (possible phosphate-induced osteomalacia) could occur. The following adverse effects may be observed (primarily from sodium or potassium): headaches; dizziness; mental confusion; seizures; weakness or heaviness of legs; unusual tiredness or weakness; muscle cramps; numbness, tingling, pain, or weakness of hands or feet; numbness or tingling around lips; fast or irregular heartbeat; shortness of breath or troubled breathing; swelling of feet or lower legs; unusual weight gain; low urine output; unusual thirst.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
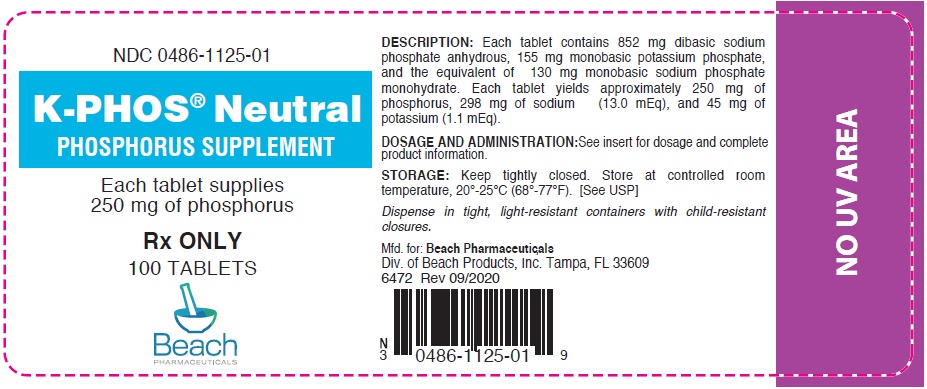
PRINCIPAL DISPLAY PANEL - 250 mg Tablet Bottle Label
NDC 0486-1125-01
K-PHOS® Neutral
PHOSPHORUS SUPPLEMENTEach tablet supplies
250 mg of phosphorusRx ONLY
100 TABLETS
Beach
PHARMACEUTICALS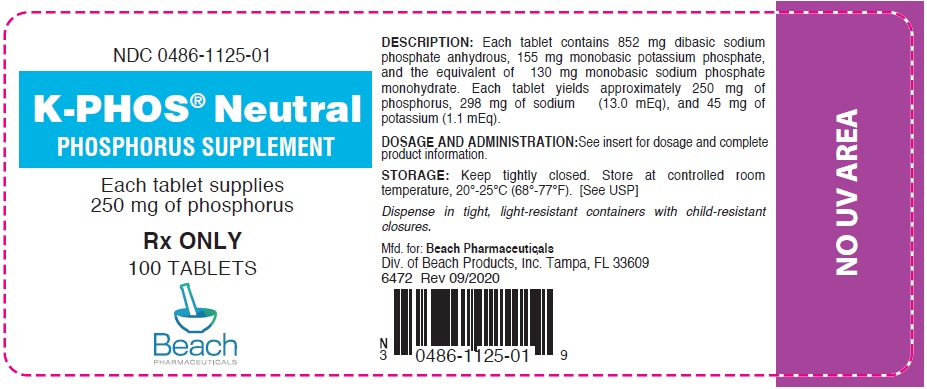
PRINCIPAL DISPLAY PANEL - 250 mg Tablet Bottle Label
NDC 0486-1125-05
K-PHOS® Neutral
PHOSPHORUS SUPPLEMENTEach tablet supplies
250 mg of phosphorusRx ONLY
500 TABLETS
Beach
PHARMACEUTICALS
-
INGREDIENTS AND APPEARANCE
K PHOS NEUTRAL
sodium phosphate, dibasic, anhydrous, potassium phosphate, monobasic, and sodium phosphate, monobasic, monohydrate tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0486-1125 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) (PHOSPHATE ION - UNII:NK08V8K8HR, SODIUM CATION - UNII:LYR4M0NH37) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS 852 mg POTASSIUM PHOSPHATE, MONOBASIC (UNII: 4J9FJ0HL51) (PHOSPHATE ION - UNII:NK08V8K8HR, POTASSIUM CATION - UNII:295O53K152) POTASSIUM PHOSPHATE, MONOBASIC 155 mg SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE (UNII: 593YOG76RN) (PHOSPHATE ION - UNII:NK08V8K8HR, SODIUM CATION - UNII:LYR4M0NH37) SODIUM PHOSPHATE, MONOBASIC, MONOHYDRATE 130 mg Inactive Ingredients Ingredient Name Strength POVIDONE K29/32 (UNII: 390RMW2PEQ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) MAGNESIUM STEARATE (UNII: 70097M6I30) STEARIC ACID (UNII: 4ELV7Z65AP) HYPROMELLOSES (UNII: 3NXW29V3WO) SODIUM CITRATE (UNII: 1Q73Q2JULR) MALTODEXTRIN (UNII: 7CVR7L4A2D) TRIACETIN (UNII: XHX3C3X673) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) Product Characteristics Color white Score 2 pieces Shape OVAL Size 19mm Flavor Imprint Code Beach;11;25 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0486-1125-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 04/11/1977 2 NDC:0486-1125-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 04/11/1977 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/11/1977 Labeler - Beach Products, Inc. (032763633) Establishment Name Address ID/FEI Business Operations LGM Pharma Solutions, LLC 117549200 manufacture(0486-1125)