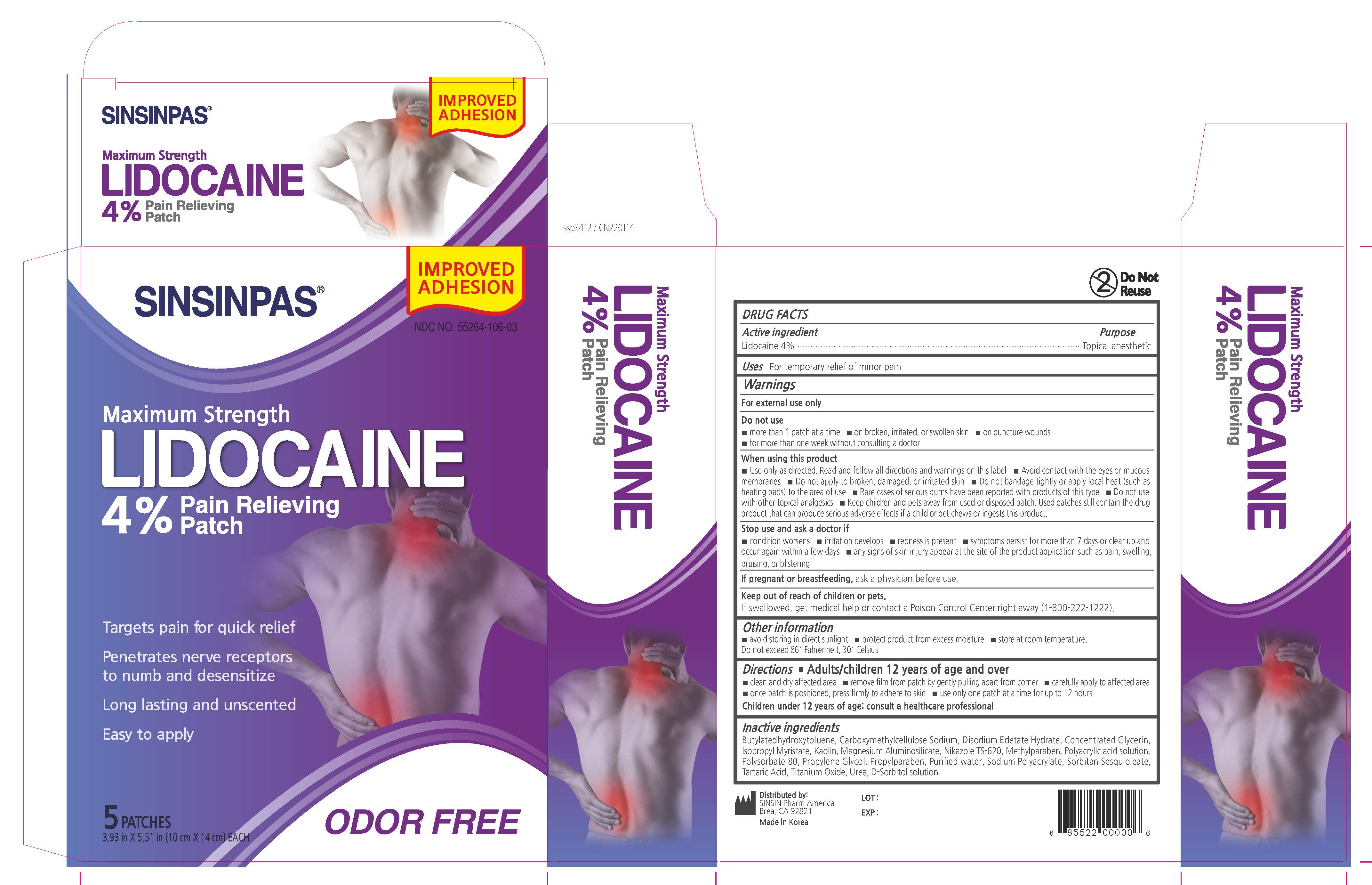
Label: SINSINPAS LIDOCAINE PAIN RELIEVING- lidocaine patch
- NDC Code(s): 55264-106-01, 55264-106-02, 55264-106-03
- Packager: Sinsin Pharmaceutical Co., Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated March 12, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Uses
- Warnings
- Do not use
-
When using this product
Use only as directed, Read and follow all directions and warnings on this label
Avoid contact with the eyes or mucous membranes - Do not apply to broken, damaged, or irritated skin Do not bandage tightly or apply local heat (such as heating pads) to the area of use
Rare cases of serious burns have been reported with products of this type
Do not use with other topical analgesics
Keep children and pets away from used or disposed patch. Used patches still contain the drug product that can produce serious adverse effects if a child or pet chews or ingests this product.
- Stop use and ask a doctor if
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- Other information
- Directions
- Ask a doctor
-
Inactive ingredients
butylated hydroxytoluene, carboxymethylcellulose sodium, Concentrated Glycerin, Disodium edetate Hydrate
isopropyl myristate, kaolin, magnesium aluminosilicate, Nikazole TS-620,
methyl paraben, polyacrylic acid solution, polysorbate 80, propylene glycol, propyl paraben, purified water,
sodium polyacrylate, sorbitan sesquioleate, tartaric acid, titanium dioxide, urea, D-sorbitol solution,
- Purpose
- Uses
- Do not use
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SINSINPAS LIDOCAINE PAIN RELIEVING
lidocaine patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55264-106 Route of Administration TRANSDERMAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 560 mg in 14 g Inactive Ingredients Ingredient Name Strength ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color white Score Shape RECTANGLE Size 14mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55264-106-03 5 in 1 BOX 05/25/2017 02/04/2022 1 NDC:55264-106-02 1 in 1 POUCH 1 NDC:55264-106-01 14 g in 1 PATCH; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/25/2017 12/30/2049 Labeler - Sinsin Pharmaceutical Co., Ltd (823149161) Registrant - Sinsin Pharmaceutical Co., Ltd. (687867143)