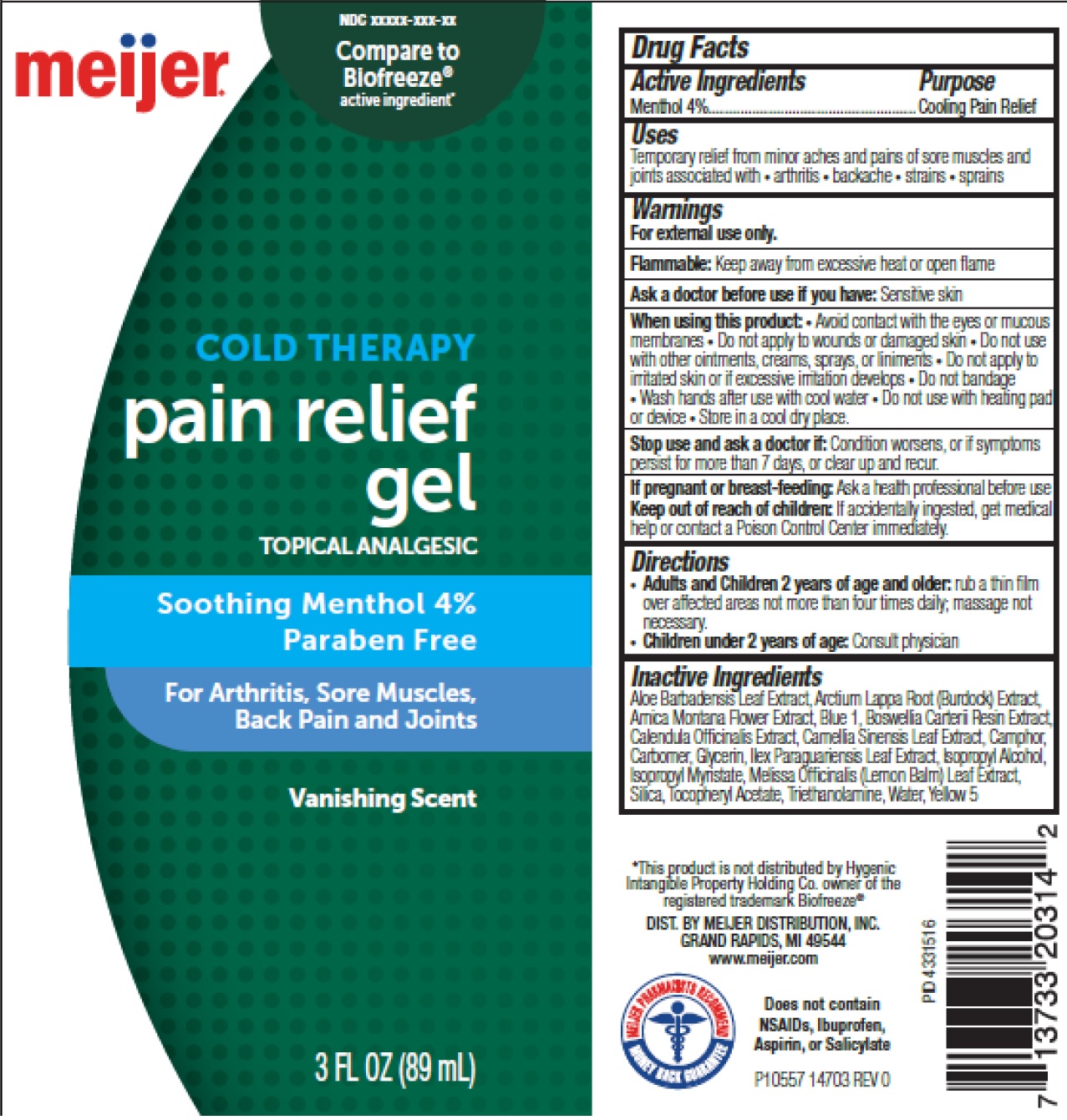
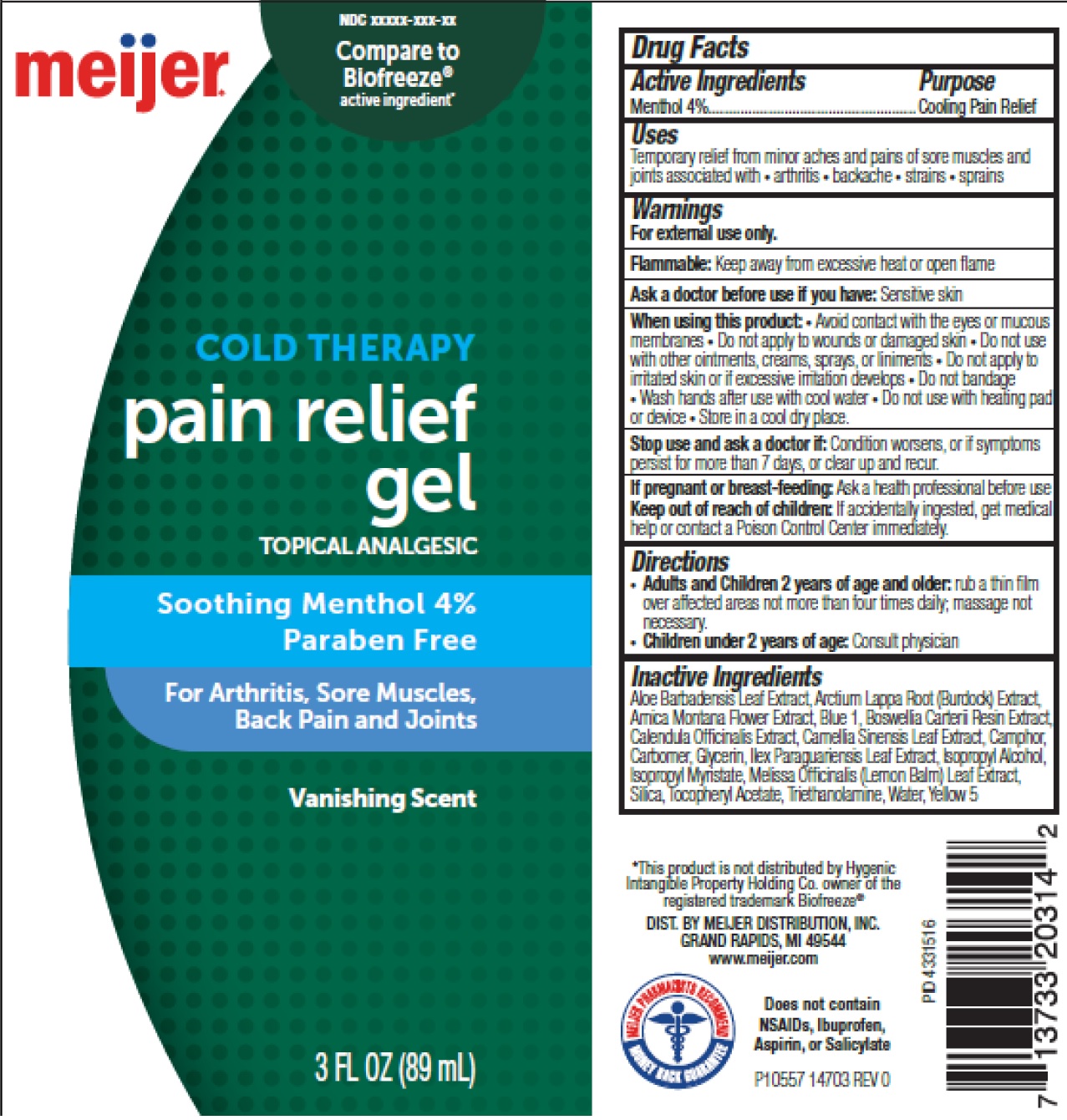
Label: MEIJER COLD THERAPY PAIN RELIEF- menthol gel
- NDC Code(s): 41250-864-25
- Packager: Meijer Distribution, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
-
Warnings
For external use only.
Flammable: Keep away from excessive heat or open flame
When using this product:
• Avoid contact with the eyes or mucous membranes • Do not apply to wounds or damaged skin • Do not use with other ointments, creams, sprays, or liniments • Do not apply to irritated skin or if excessive irritattion develops • Do not bandage • Wash hands after use with cool water
- Directions
-
Inactive Ingredients
Aloe Barbadensis Leaf Extract, Arctium Lappa Root (Burdock) Extract, Arnica Montana Flower Extract, Blue 1, Boswellia Carterii Resin Extract, Calendula Officinalis Extract, Camellia Sinensis Leaf Extract, Camphor, Carbomer, Glycerin, Ilex Paraguariensis Leaf Extract, Isopropyl Alcohol, Isopropyl Myristate, Melissa Officinalis (Lemon Balm) Leaf Extract, Silica, Tocopheryl Acetate, Triethanolamine, Water, Yellow 5
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
MEIJER COLD THERAPY PAIN RELIEF
menthol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41250-864 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) ISOPROPYL ALCOHOL (UNII: ND2M416302) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) MELISSA OFFICINALIS LEAF (UNII: 50D2ZE9219) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41250-864-25 89 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/22/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 10/22/2018 12/31/2025 Labeler - Meijer Distribution, Inc. (006959555) Establishment Name Address ID/FEI Business Operations Span Packaging Services LLC 117101131 manufacture(41250-864) Establishment Name Address ID/FEI Business Operations Cosmetic Essence, LLC 825646862 manufacture(41250-864)