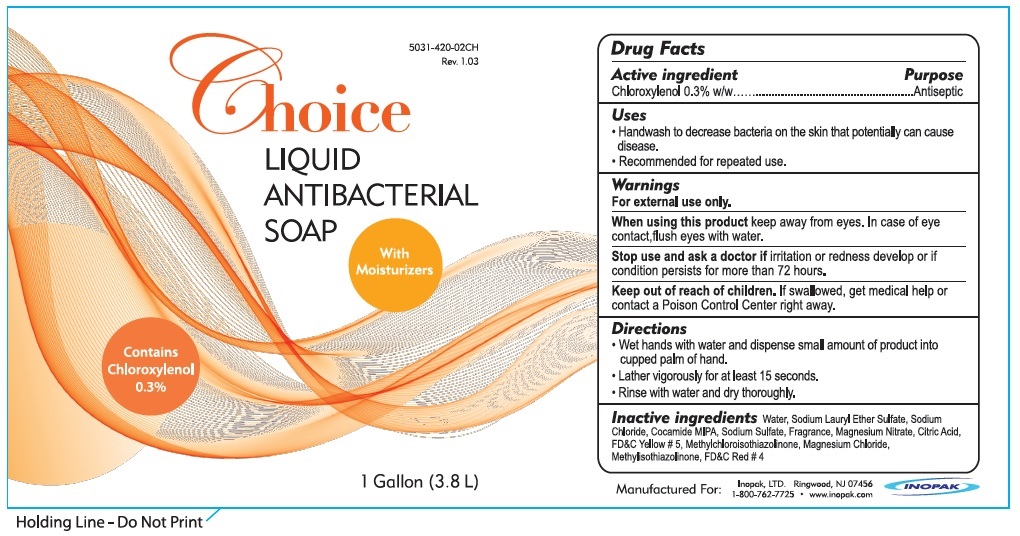
Label: INODERM CHOICE ANTIBACTERIAL- chloroxylenol liquid
- NDC Code(s): 73062-523-42
- Packager: Avro Enterprises LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Inactive ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
INODERM CHOICE ANTIBACTERIAL
chloroxylenol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73062-523 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLOROXYLENOL (UNII: 0F32U78V2Q) (CHLOROXYLENOL - UNII:0F32U78V2Q) CHLOROXYLENOL 3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) SODIUM CHLORIDE (UNII: 451W47IQ8X) COCO MONOISOPROPANOLAMIDE (UNII: 21X4Y0VTB1) SODIUM SULFATE (UNII: 0YPR65R21J) MAGNESIUM NITRATE (UNII: 77CBG3UN78) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) FD&C RED NO. 4 (UNII: X3W0AM1JLX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73062-523-42 3800 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/14/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 04/14/2023 Labeler - Avro Enterprises LLC (804030166)