Label: BAREMINERALS ORIGINAL FOUNDATION BROAD SPECTRUM SPF 15 MEDIUM DARK 23- titanium dioxide and zinc oxide powder
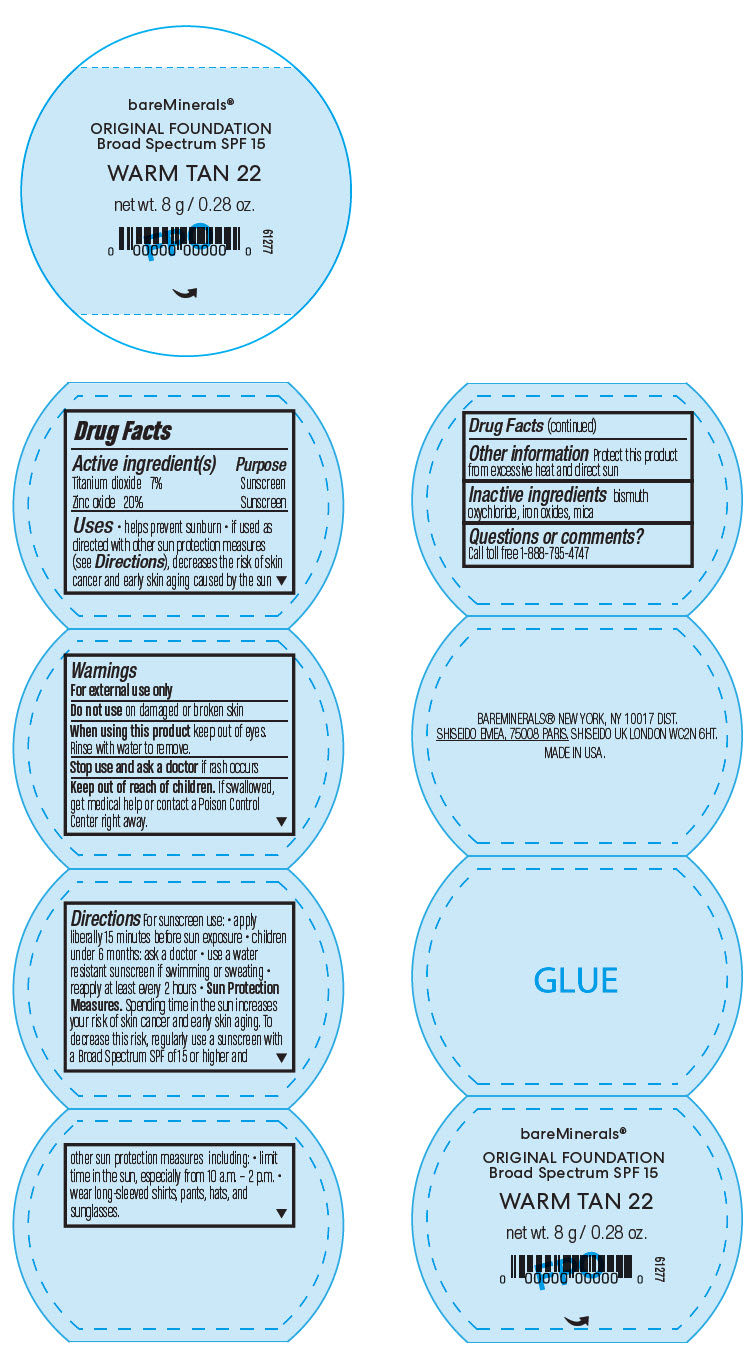
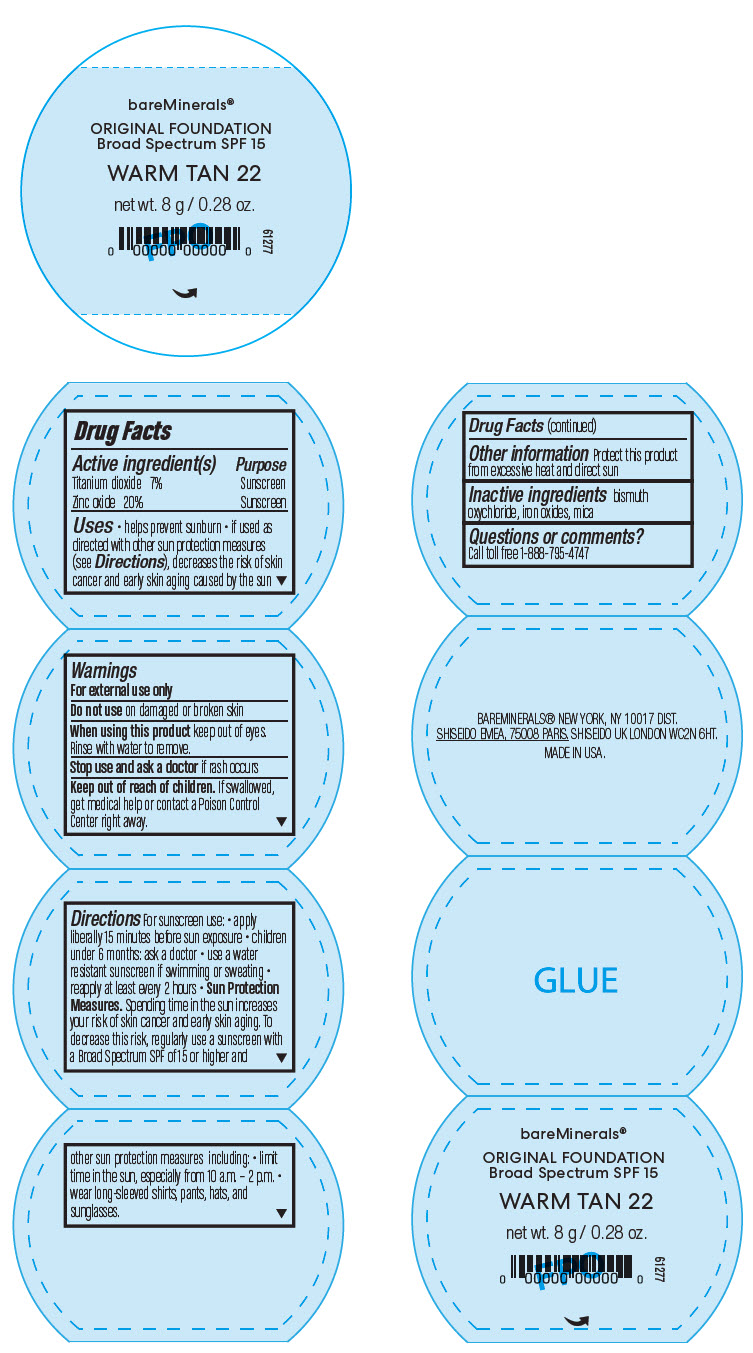
BAREMINERALS ORIGINAL FOUNDATION BROAD SPECTRUM SPF 15 WARM TAN 22- titanium dioxide and zinc oxide powder
BAREMINERALS ORIGINAL FOUNDATION BROAD SPECTRUM SPF 15 GOLDEN DARK 25- titanium dioxide and zinc oxide powder
BAREMINERALS ORIGINAL FOUNDATION BROAD SPECTRUM SPF 15 WARM DARK 26- titanium dioxide and zinc oxide powder
BAREMINERALS ORIGINAL FOUNDATION BROAD SPECTRUM SPF 15 WARM DEEP 27- titanium dioxide and zinc oxide powder
-
NDC Code(s):
98132-015-08,
98132-016-08,
98132-020-08,
98132-021-02, view more98132-021-08, 98132-021-75, 98132-022-02, 98132-022-08, 98132-022-75
- Packager: Orveon Global US LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- children under 6 months: ask a doctor
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses.
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 8 g Jar Label - Medium Dark 23
- PRINCIPAL DISPLAY PANEL - 8 g Jar Label - Warm Tan 22
- PRINCIPAL DISPLAY PANEL - 8 g Jar Label - Golden Dark 25
- PRINCIPAL DISPLAY PANEL - 8 g Jar Label - Warm Dark 26
- PRINCIPAL DISPLAY PANEL - 8 g Jar Label - Warm Deep 27
-
INGREDIENTS AND APPEARANCE
BAREMINERALS ORIGINAL FOUNDATION BROAD SPECTRUM SPF 15 MEDIUM DARK 23
titanium dioxide and zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 560 mg in 8 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1600 mg in 8 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-015-08 8 g in 1 JAR; Type 0: Not a Combination Product 08/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/01/2021 BAREMINERALS ORIGINAL FOUNDATION BROAD SPECTRUM SPF 15 WARM TAN 22
titanium dioxide and zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 560 mg in 8 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1600 mg in 8 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-016-08 8 g in 1 JAR; Type 0: Not a Combination Product 08/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/01/2021 BAREMINERALS ORIGINAL FOUNDATION BROAD SPECTRUM SPF 15 GOLDEN DARK 25
titanium dioxide and zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-020 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 560 mg in 8 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1600 mg in 8 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-020-08 8 g in 1 JAR; Type 0: Not a Combination Product 08/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/01/2021 BAREMINERALS ORIGINAL FOUNDATION BROAD SPECTRUM SPF 15 WARM DARK 26
titanium dioxide and zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-021 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 560 mg in 8 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1600 mg in 8 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-021-08 8 g in 1 JAR; Type 0: Not a Combination Product 08/01/2021 2 NDC:98132-021-02 2 g in 1 JAR; Type 0: Not a Combination Product 08/01/2021 3 NDC:98132-021-75 0.75 g in 1 JAR; Type 0: Not a Combination Product 08/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/01/2021 BAREMINERALS ORIGINAL FOUNDATION BROAD SPECTRUM SPF 15 WARM DEEP 27
titanium dioxide and zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:98132-022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 560 mg in 8 g ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 1600 mg in 8 g Inactive Ingredients Ingredient Name Strength MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) BISMUTH OXYCHLORIDE (UNII: 4ZR792I587) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:98132-022-08 8 g in 1 JAR; Type 0: Not a Combination Product 08/01/2021 2 NDC:98132-022-02 2 g in 1 JAR; Type 0: Not a Combination Product 08/01/2021 3 NDC:98132-022-75 0.75 g in 1 JAR; Type 0: Not a Combination Product 08/01/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 08/01/2021 Labeler - Orveon Global US LLC (118344494)