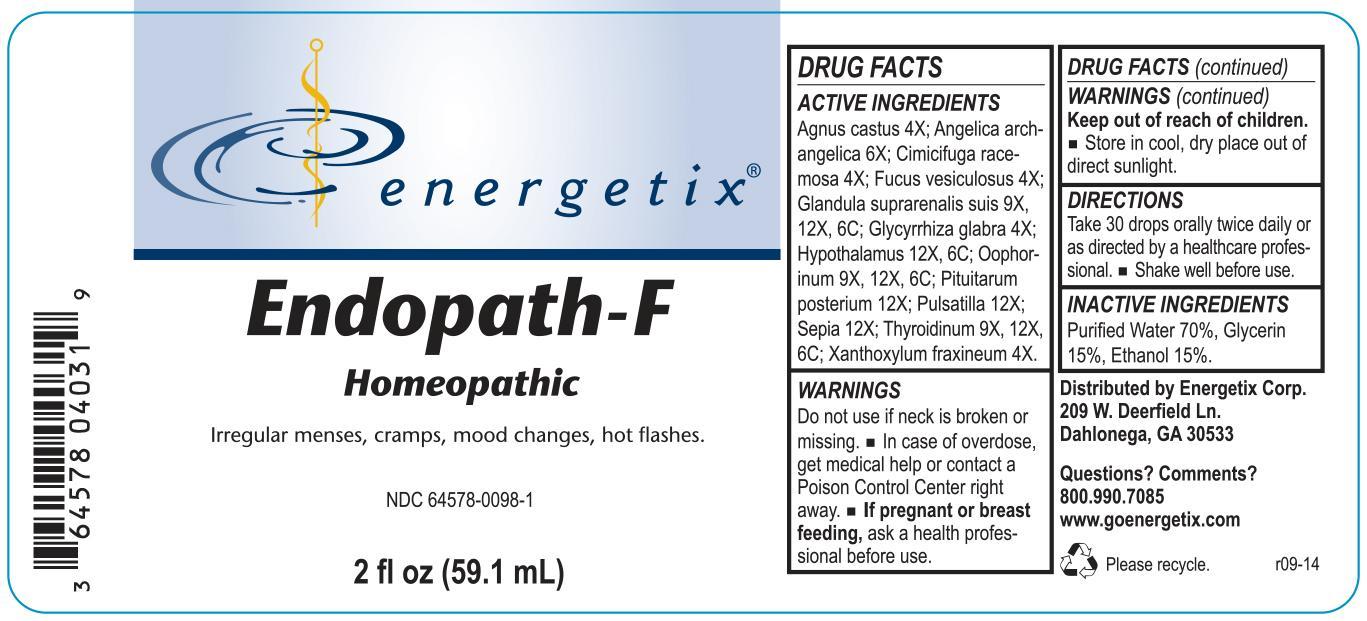
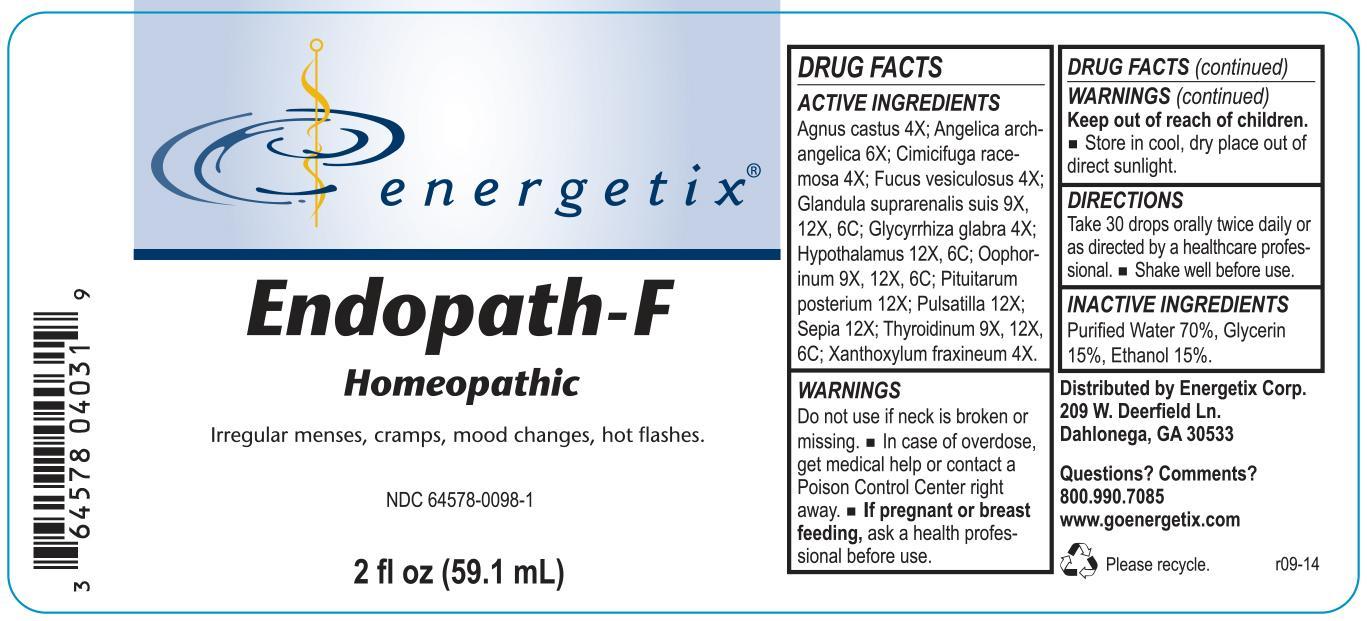
Label: ENDOPATH-F- homeopathic liquid liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 64578-0098-1 - Packager: Energetix Corp
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated April 2, 2014
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PRINCIPAL DISPLAY PANEL
-
ACTIVE INGREDIENT
Agnus castus 4X; Angelica archangelica 6X; Cimicifuga racemosa 4X; Fucus vesiculosus 4X; Glandula suprarenalis suis 9X, 12X, 6C; Glycyrrhiza glabra 4X; Hypothalamus 12X, 6C; Oophorinum 9X, 12X, 6C; Pituitarum posterium 12X; Pulsatilla 12X; Sepia 12X; Thyroidinum 9X, 12X, 6C; Xanthoxylum fraxineum 4X.
- WARNINGS
- DO NOT USE
- OVERDOSAGE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- QUESTIONS
- PURPOSE
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
INGREDIENTS AND APPEARANCE
ENDOPATH-F
homeopathic liquid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64578-0098 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SUS SCROFA ADRENAL GLAND (UNII: 398IYQ16YV) (SUS SCROFA ADRENAL GLAND - UNII:398IYQ16YV) SUS SCROFA ADRENAL GLAND 9 [hp_X] in 59.1 mL CHASTE TREE (UNII: 433OSF3U8A) (CHASTE TREE - UNII:433OSF3U8A) CHASTE TREE 4 [hp_X] in 59.1 mL BLACK COHOSH (UNII: K73E24S6X9) (BLACK COHOSH - UNII:K73E24S6X9) BLACK COHOSH 4 [hp_X] in 59.1 mL FUCUS VESICULOSUS (UNII: 535G2ABX9M) (FUCUS VESICULOSUS - UNII:535G2ABX9M) FUCUS VESICULOSUS 4 [hp_X] in 59.1 mL GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) (GLYCYRRHIZA GLABRA - UNII:2788Z9758H) GLYCYRRHIZA GLABRA 4 [hp_X] in 59.1 mL BOS TAURUS HYPOTHALAMUS (UNII: S6G2NLH4Y7) (BOS TAURUS HYPOTHALAMUS - UNII:S6G2NLH4Y7) BOS TAURUS HYPOTHALAMUS 12 [hp_X] in 59.1 mL ANGELICA ARCHANGELICA ROOT (UNII: DTN01M69SN) (ANGELICA ARCHANGELICA ROOT - UNII:DTN01M69SN) ANGELICA ARCHANGELICA ROOT 6 [hp_X] in 59.1 mL SUS SCROFA OVARY (UNII: S7YTV04R8O) (SUS SCROFA OVARY - UNII:S7YTV04R8O) SUS SCROFA OVARY 9 [hp_X] in 59.1 mL PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 12 [hp_X] in 59.1 mL SEPIA OFFICINALIS JUICE (UNII: QDL83WN8C2) (SEPIA OFFICINALIS JUICE - UNII:QDL83WN8C2) SEPIA OFFICINALIS JUICE 12 [hp_X] in 59.1 mL THYROID, UNSPECIFIED (UNII: 0B4FDL9I6P) (THYROID, UNSPECIFIED - UNII:0B4FDL9I6P) THYROID, UNSPECIFIED 9 [hp_X] in 59.1 mL ZANTHOXYLUM AMERICANUM BARK (UNII: A4KL1HMZ7T) (ZANTHOXYLUM AMERICANUM BARK - UNII:A4KL1HMZ7T) ZANTHOXYLUM AMERICANUM BARK 4 [hp_X] in 59.1 mL SUS SCROFA PITUITARY GLAND (UNII: E8S87O660T) (SUS SCROFA PITUITARY GLAND - UNII:E8S87O660T) SUS SCROFA PITUITARY GLAND 12 [hp_X] in 59.1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) 8.862 mL in 59.1 mL GLYCERIN (UNII: PDC6A3C0OX) 8.862 mL in 59.1 mL WATER (UNII: 059QF0KO0R) 41.356 mL in 59.1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64578-0098-1 59.1 mL in 1 BOTTLE, DROPPER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 10/06/2014 Labeler - Energetix Corp (969572502)