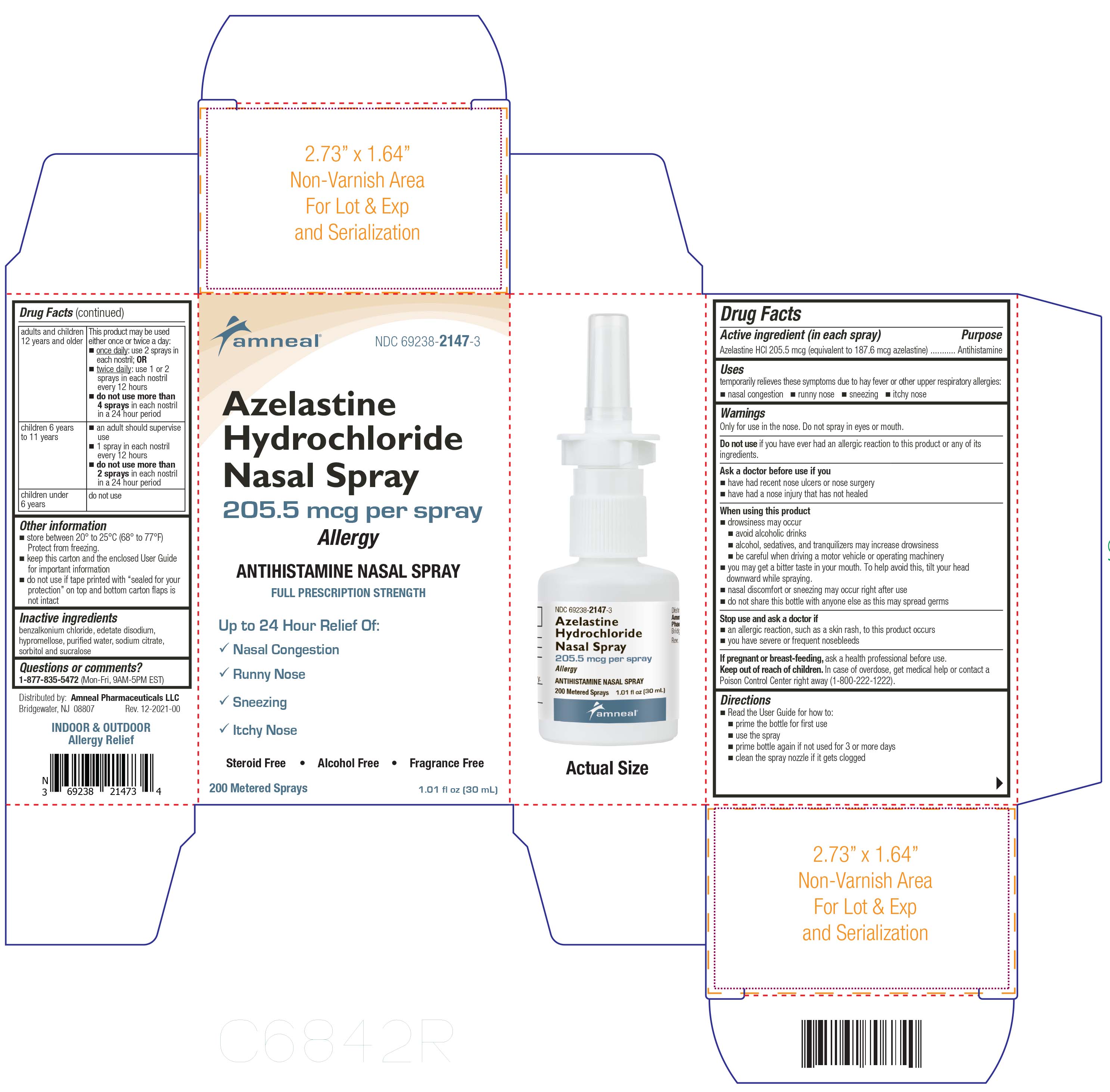
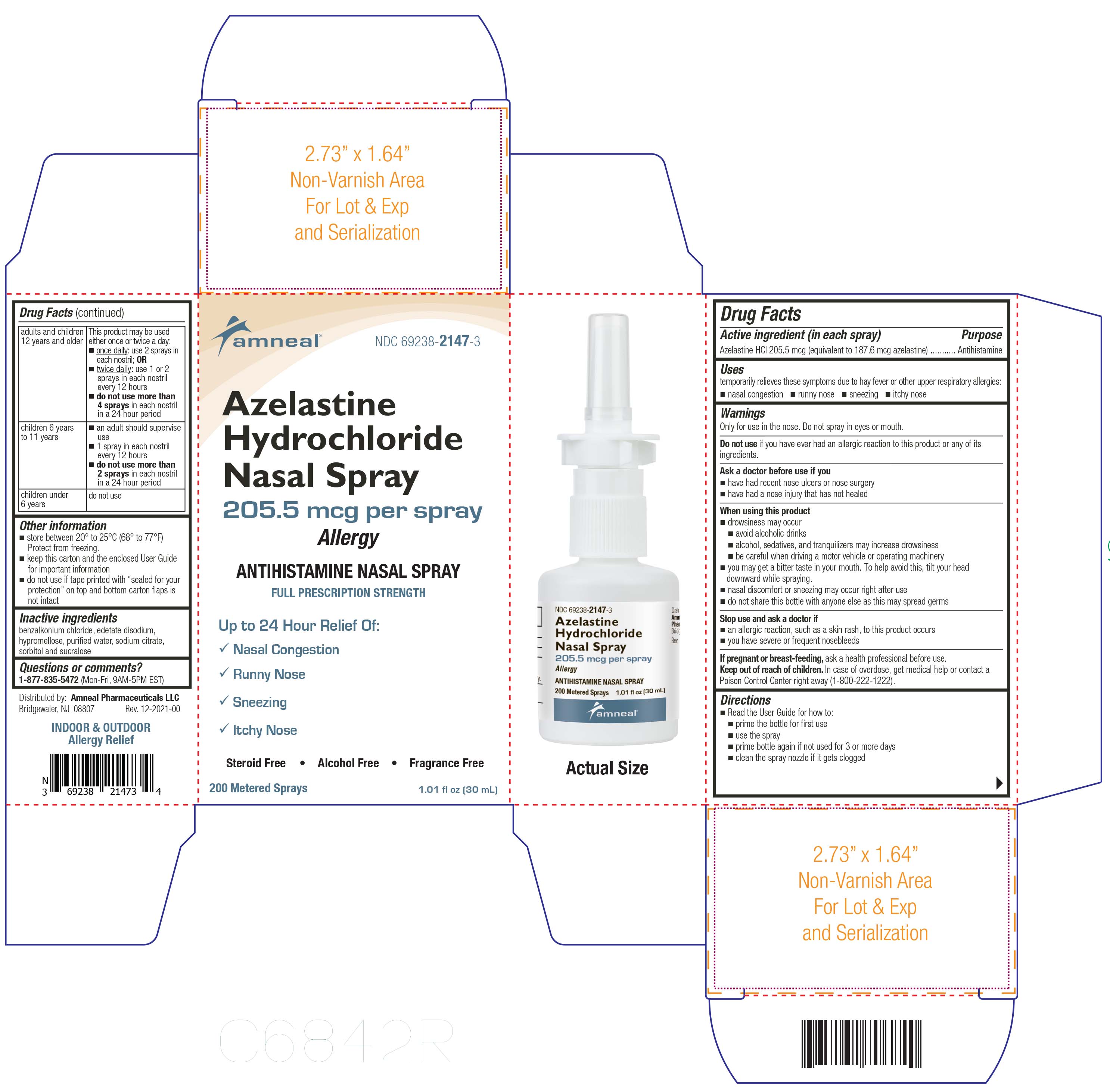
Label: AZELASTINE HCL- azelastine spray, metered
- NDC Code(s): 69238-2147-3
- Packager: Amneal Pharmaceuticals NY LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each spray)
- Purpose
- Uses
-
Warnings
Only for use in the nose. Do not spray in eyes or mouth.
Do not use
Do not use if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if
Ask a doctor before use if you
- have had recent nose ulcers or nose surgery
- have had a nose injury that has not healed
When using this product
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving motor vehicle or operation machinery
- you may get a bitter taste in your mouth. To help avoid this, tilt your head downward while spraying.
- nasal discomfort or sneezing may occur right after use
- do not share this bottle with anyone else as this may spread germs
-
Directions
Directions
- Read the User Guide for how to:
- prime the bottle before first use
- use the spray
- prime bottle again if not used for 3 or more days
- clean the spray nozzle if it gets clogged
adults and children 12 years and older
This product may be used either once or twice a day:
■once daily: use 2 sprays in each nostril; OR
■twice daily: use 1 or 2 sprays in each nostril every 12 hours
■do not use more than 4 sprays in each nostril in a 24 hour period
children 6 years to 11 years
■an adult should supervise use
■1 spray in each nostril every 12 hours
■do not use more than 2 sprays in each nostril in a 24 hour period
children under 6 years
do not use
- Read the User Guide for how to:
- Other information
- Inactive ingredients
- Questions
-
User Guide
Azelastine HCl Nasal Spray (205.5 mcg per spray)
HOW TO START GETTING STEROID-FREE ALLERGY RELIEF RIGHT NOW
This User Guide will give you detailed directions on how to prime the bottle, use the spray, and how to clean a clogged nozzle.
Keep this User Guide as it contains important information.
GET THE BOTTLE READY
Remove the dust cover over the tip of the bottle
and the white safety clip just under
the shoulders of the bottle before using.
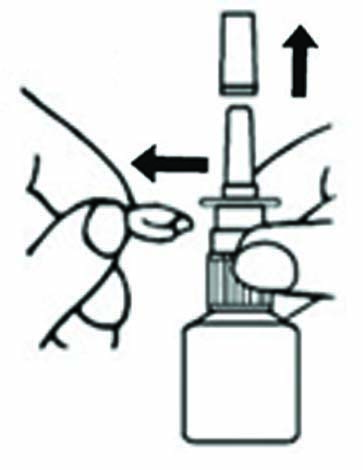
PRIME THE BOTTLE
WHEN TO PRIME- Before you use Azelastine HCl Nasal Spray for the first time
- If you have not used Azelastine HCl Nasal Spray for 3 or more days
- After you clean a clogged nozzle
HOW TO PRIME
- Aim bottle away from face.
- Hold bottle as shown and pump until a fine mist appears.
- If a fine mist does not appear after 6 sprays, your nozzle may be clogged. Please see “How to Clean a Clogged Nozzle” section.
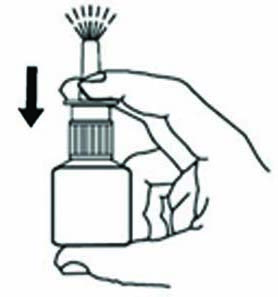
HOW TO USE
Important: For use in your nose onlySTEP 1
Blow your nose to clear your nostrils.
STEP 2
Tilt your head downward toward your toes.
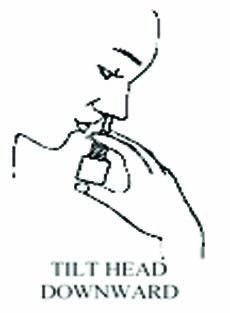
STEP 3
Hold bottle with thumb under bottle and
spray nozzle between fingers.
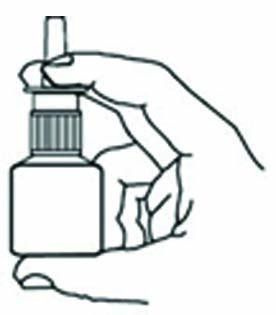
STEP 4
Close one nostril.
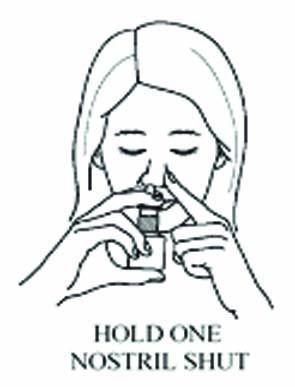
STEP 5
Put the spray tip about ¼ inch to ½ inch into the other nostril.
Hold bottle upright and aim the spray tip toward the back of your nose.
STEP 6
While sniffing gently, press the pump once or twice
(according to dosing directions found in Table 1).
Repeat Steps 4, 5 and 6 in other nostril.
STEP 7
Do not tilt your head back immediately after using Azelastine HCl Nasal Spray.
This will help to keep the medicine from going into your throat,
which may cause a bitter taste.
STEP 8
When you finish using your Azelastine HCl Nasal Spray, wipe the spray tip
with a clean tissue or cloth. Put the clip and cap back on the bottle.
FOR USE IN CHILDREN
An adult should supervise use.
(See Steps 1 through 8).
SELECT THE DOSE DIRECTIONS
Do not use more than directed
TABLE 1
adults & children 12 years and older
This product may be used either once or twice a day:
■once daily: use 2 sprays in each nostril; OR
■twice daily: use 1 or 2 sprays in each nostril every 12 hours
■do not use more than 4 sprays in each nostril in a 24 hour period
children 6 years to 11 years
■an adult should supervise use
■1 spray in each nostril every 12 hours
■do not use more than 2 sprays in each nostril in a 24 hour period
children under 6 years
do not use
IMPORTANT INFORMATION FOR USE
Remember, when using this product:
- Drowsiness may occur*
› Avoid alcoholic drinks.
› Alcohol, sedatives, and tranquilizers may increase drowsiness.
› Be careful when driving a motor vehicle or operating machinery.
*In clinical trials, drowsiness was observed in less than 4% of patients taking Azelastine HCl Nasal Spray.
HOW TO CLEAN A CLOGGED NOZZLE -
If you cannot get your Azelastine HCl Nasal Sprayto spray a fine mist,
the nozzle may be clogged. Don’t try to unclog the nozzle
with a pin or sharp object—that can damage it.
- Unscrew the spray pump unit from the bottle
by turning it to the left (counter-clockwise).
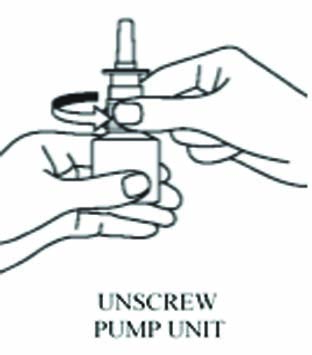
- Keep open bottle out of reach of children.
- Fill a bowl or container with warm water. Soak only the
spray pump unit in the warm water. Pump the nozzle several
times while holding it under the water to clear the clog.
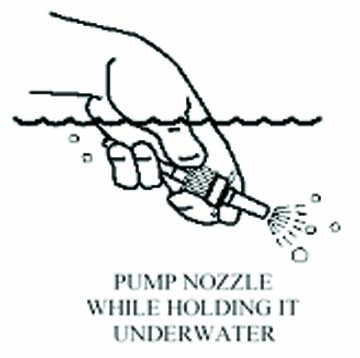
- Let the spray pump unit air dry before putting it back on.
- Tightly screw pump unit back on the open bottle
by turning clockwise (to the right).
- After cleaning, follow the instructions for priming.
QUESTIONS OR COMMENTS?
1-877-835-5472 (Mon to Fri, 9AM to 5PM EST)
Distributed by:
Amneal Pharmaceuticals LLC
Bridgewater, NJ 08807
Rev. 12-2021-00
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
AZELASTINE HCL
azelastine spray, meteredProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69238-2147 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AZELASTINE HYDROCHLORIDE (UNII: 0L591QR10I) (AZELASTINE - UNII:ZQI909440X) AZELASTINE HYDROCHLORIDE 205.5 ug Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) EDETATE DISODIUM (UNII: 7FLD91C86K) HYPROMELLOSES (UNII: 3NXW29V3WO) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) SORBITOL (UNII: 506T60A25R) HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69238-2147-3 200 in 1 BOTTLE 06/07/2024 1 1 in 1 CARTON; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA216576 06/07/2024 Labeler - Amneal Pharmaceuticals NY LLC (123797875)