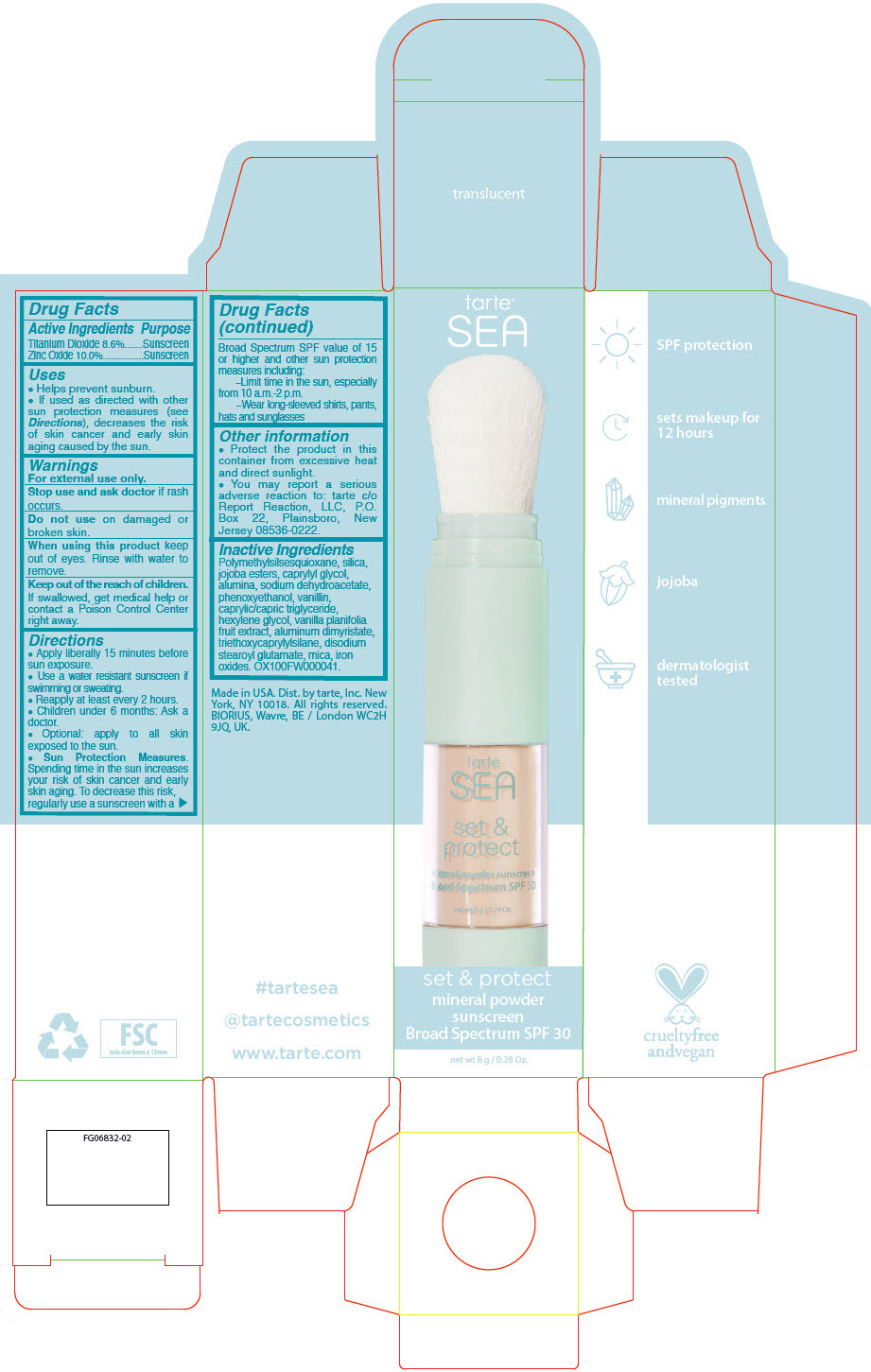
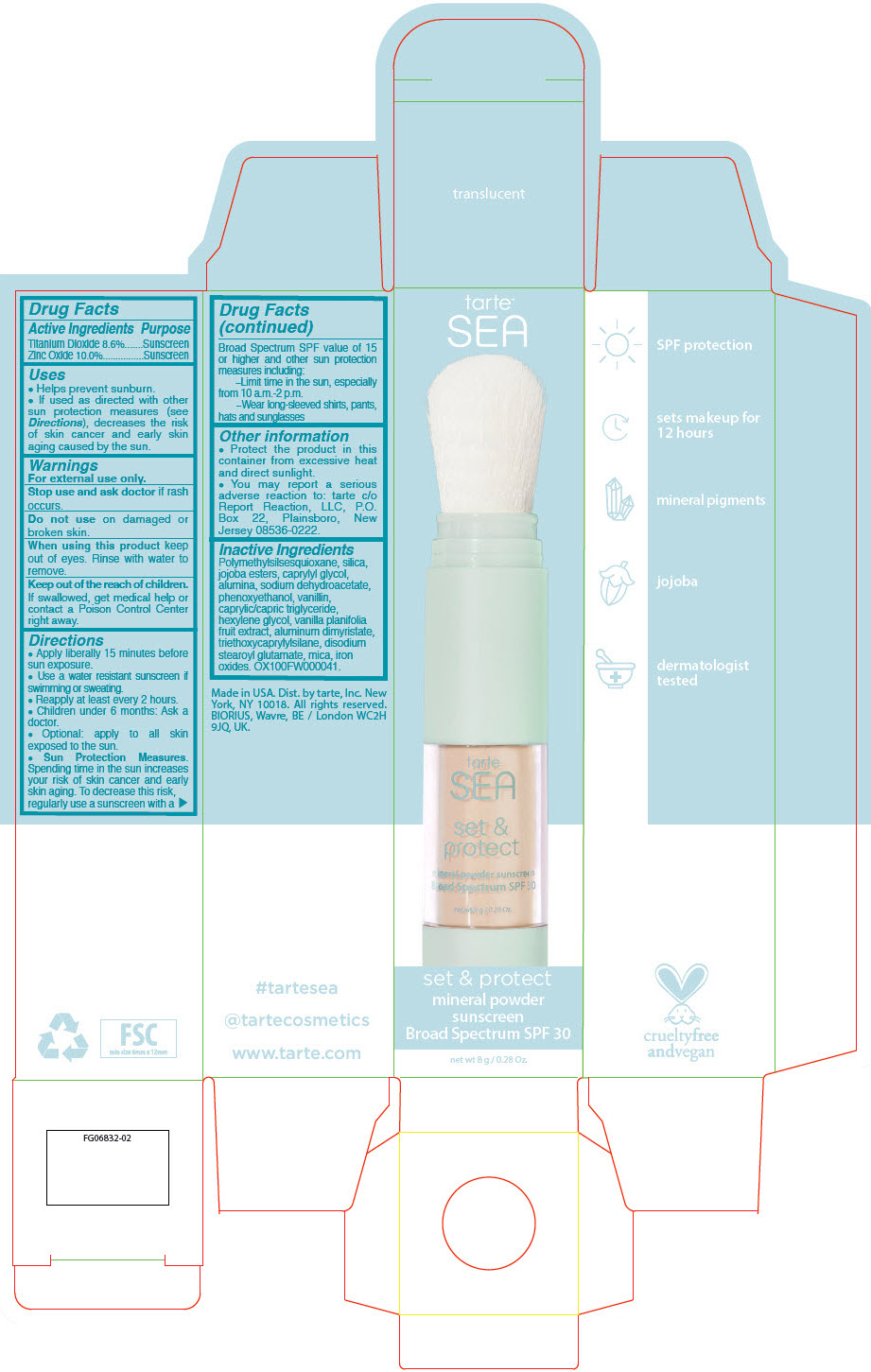
Label: SEA SET AND PROTECT MINERAL SUNSCREEN BROAD SPECTRUM SPF 30 TRANSLUCENT- titanium dioxide and zinc oxide powder
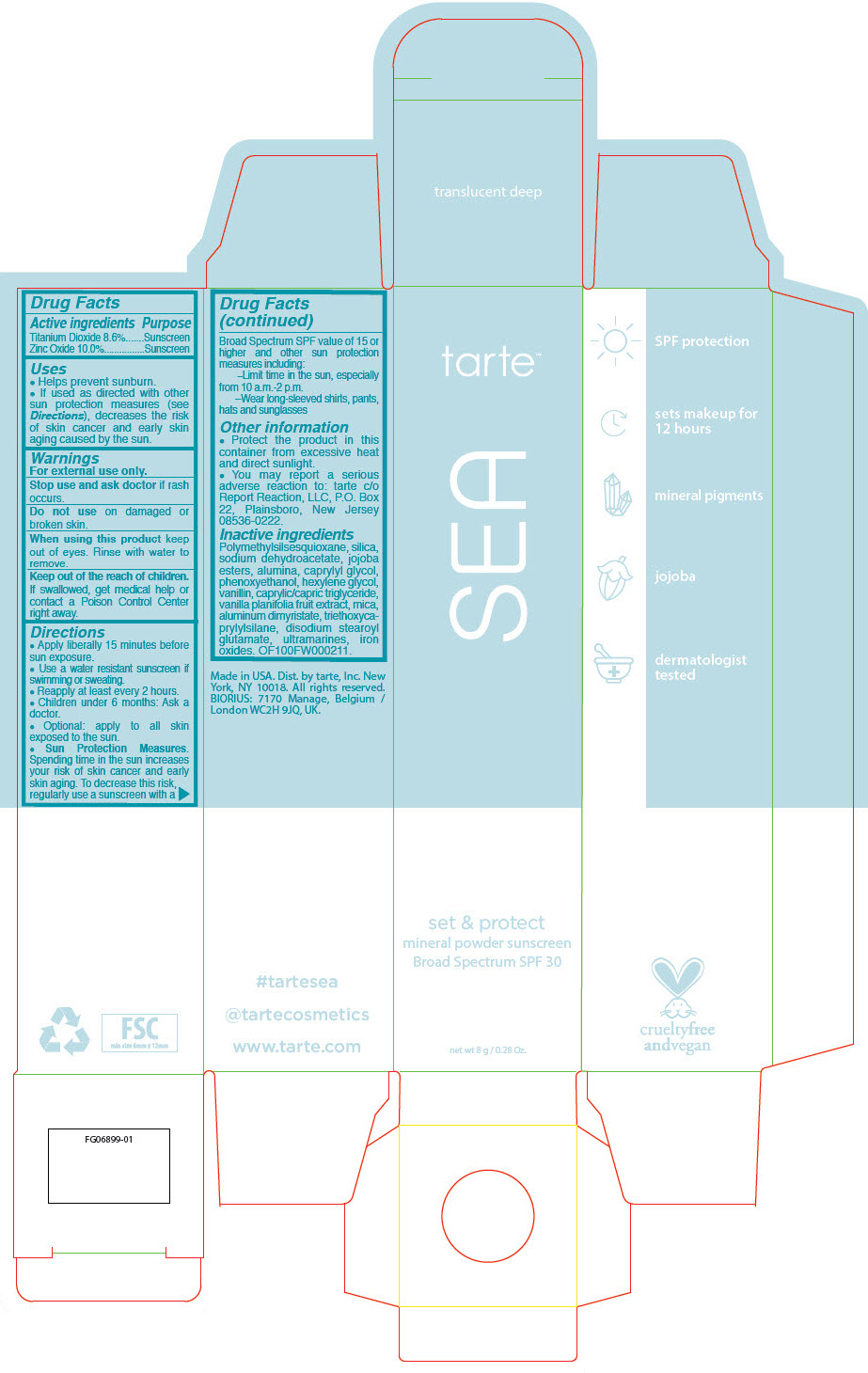
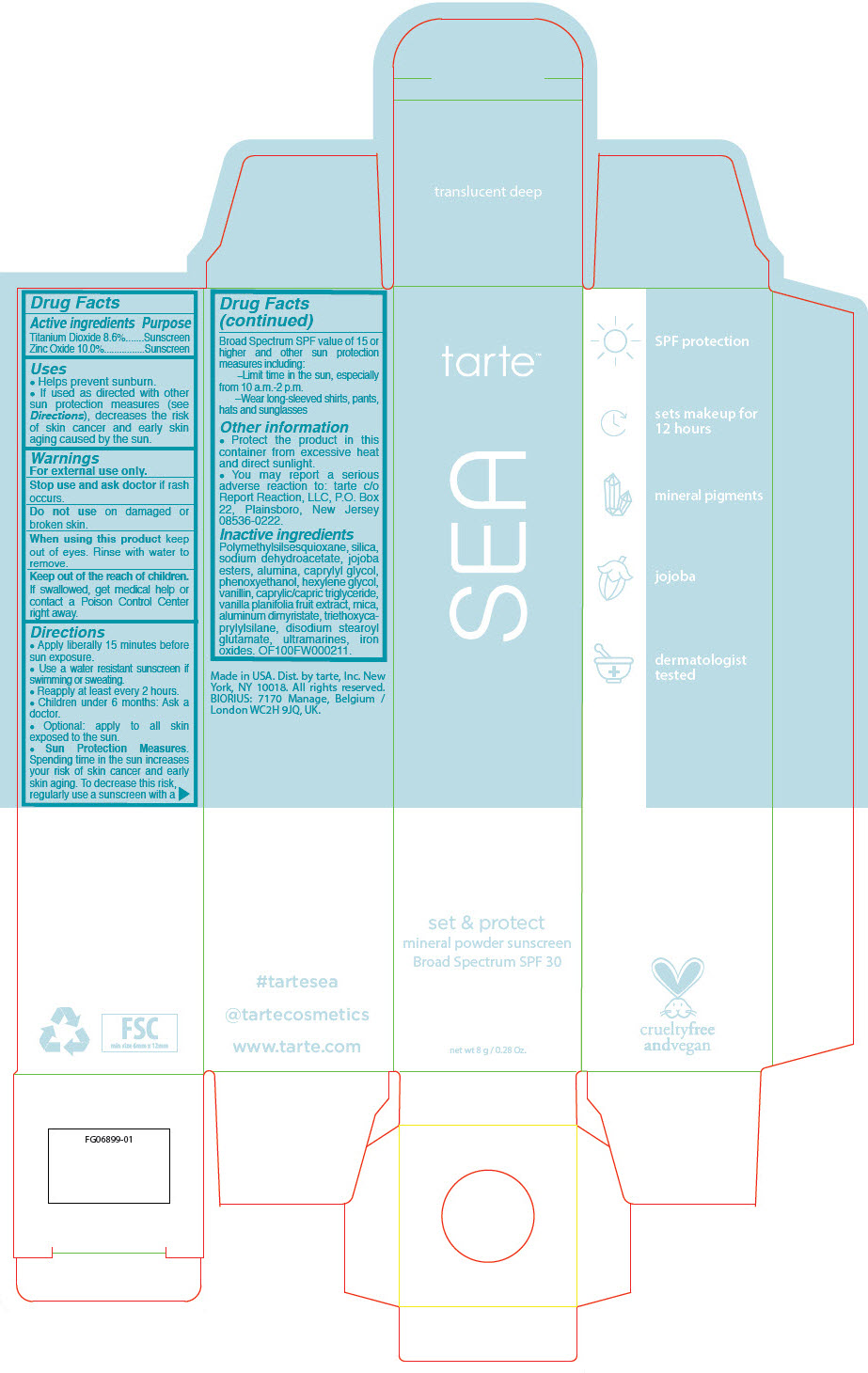
SEA SET AND PROTECT MINERAL SUNSCREEN BROAD SPECTRUM SPF 30 TRANSLUCENT DEEP- titanium dioxide and zinc oxide powder
- NDC Code(s): 51060-236-01, 51060-237-01
- Packager: Tarte, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn.
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure.
- Use a water resistant sunscreen if swimming or sweating.
- Reapply at least every 2 hours.
- Children under 6 months: Ask a doctor.
- Optional: apply to all skin exposed to the sun.
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- –
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- –
- Wear long-sleeved shirts, pants, hats and sunglasses
- Other information
-
Inactive ingredients
Polymethylsilsesquioxane, silica, jojoba esters, caprylyl glycol, alumina, sodium dehydroacetate, phenoxyethanol, vanillin, caprylic/capric triglyceride, hexylene glycol, vanilla planifolia fruit extract, aluminum dimyristate, triethoxycaprylylsilane, disodium stearoyl glutamate, mica, iron oxides. OX100FW000041.
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 8 g Tube Carton - translucent
- PRINCIPAL DISPLAY PANEL - 8 g Tube Carton - translucent deep
-
INGREDIENTS AND APPEARANCE
SEA SET AND PROTECT MINERAL SUNSCREEN BROAD SPECTRUM SPF 30 TRANSLUCENT
titanium dioxide and zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-236 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 86 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ALUMINUM OXIDE (UNII: LMI26O6933) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHENOXYETHANOL (UNII: HIE492ZZ3T) VANILLIN (UNII: CHI530446X) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) HEXYLENE GLYCOL (UNII: KEH0A3F75J) VANILLA BEAN (UNII: Q74T35078H) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-236-01 1 in 1 CARTON 03/05/2020 1 8 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 03/05/2020 SEA SET AND PROTECT MINERAL SUNSCREEN BROAD SPECTRUM SPF 30 TRANSLUCENT DEEP
titanium dioxide and zinc oxide powderProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51060-237 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 86 mg in 1 mL Zinc Oxide (UNII: SOI2LOH54Z) (Zinc Oxide - UNII:SOI2LOH54Z) Zinc Oxide 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) ALUMINUM OXIDE (UNII: LMI26O6933) CAPRYLYL GLYCOL (UNII: 00YIU5438U) PHENOXYETHANOL (UNII: HIE492ZZ3T) HEXYLENE GLYCOL (UNII: KEH0A3F75J) VANILLIN (UNII: CHI530446X) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) VANILLA BEAN (UNII: Q74T35078H) MICA (UNII: V8A1AW0880) ALUMINUM DIMYRISTATE (UNII: J2KA067N9O) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DISODIUM STEAROYL GLUTAMATE (UNII: 45ASM2L11M) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51060-237-01 1 in 1 CARTON 03/05/2020 1 8 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 03/05/2020 Labeler - Tarte, Inc. (027905186)