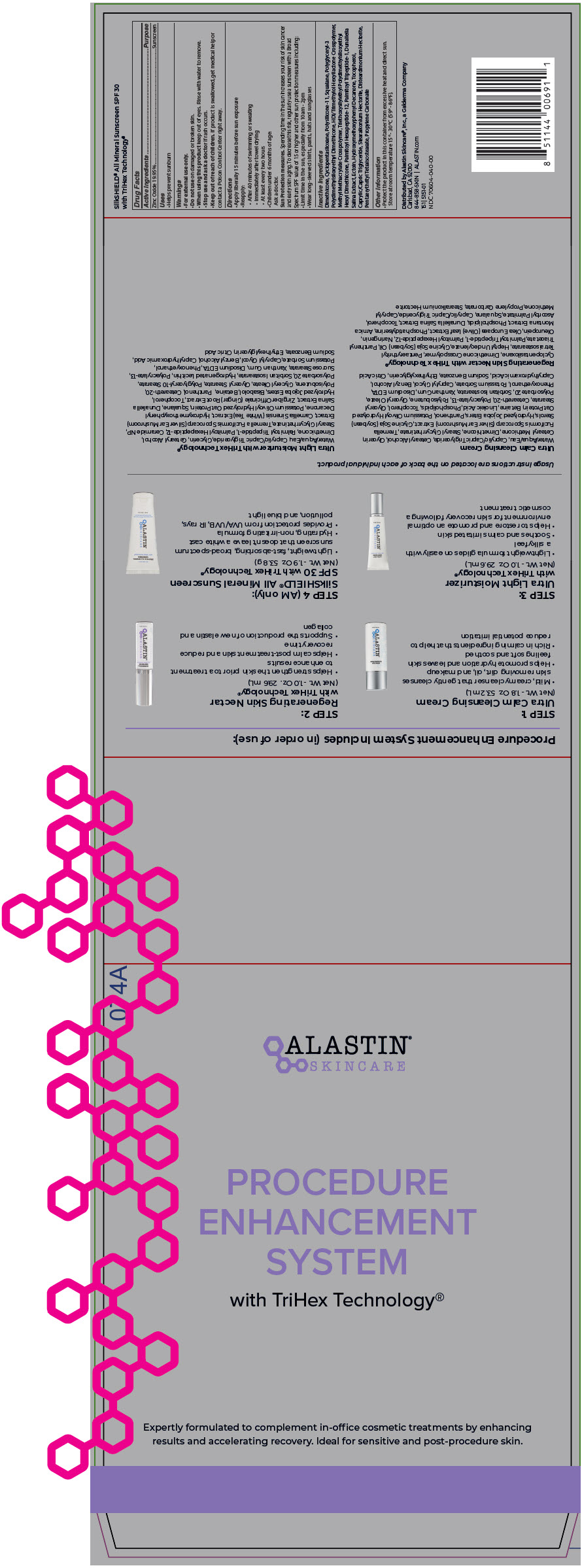
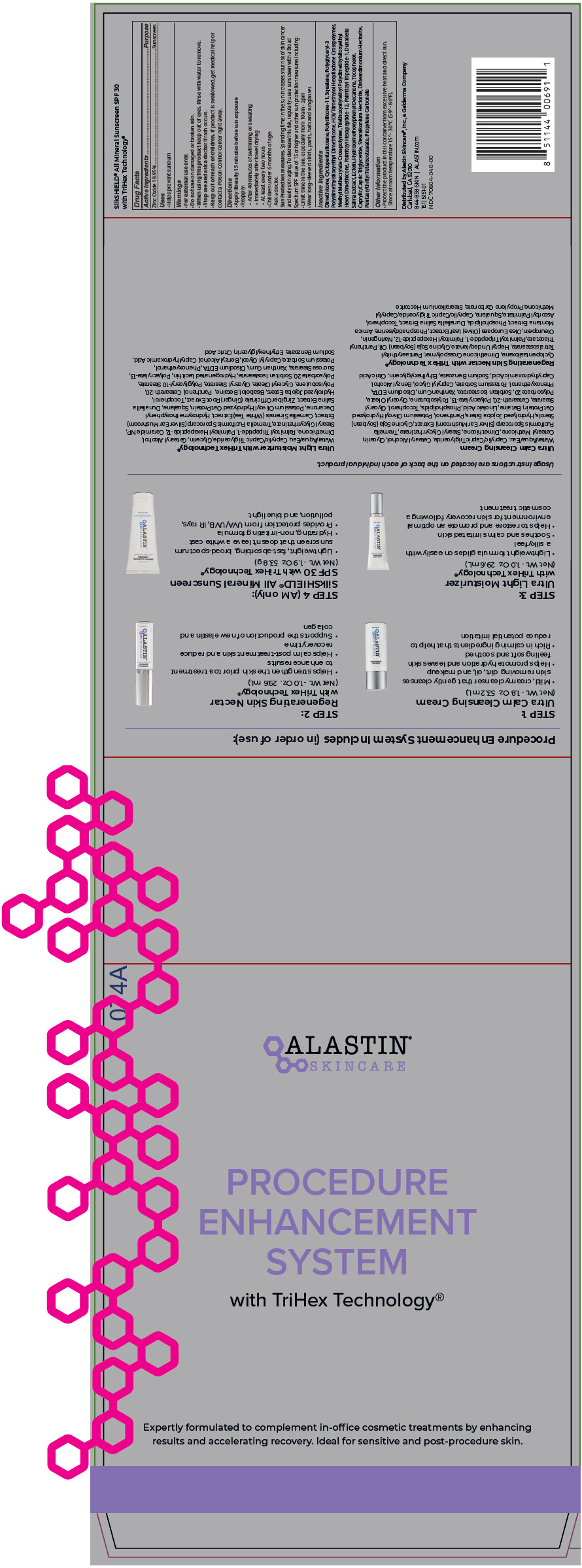
Label: PROCEDURE ENHANCEMENT SYSTEM- zinc oxide kit
- NDC Code(s): 70604-030-02, 70604-040-00
- Packager: Alastin Skincare, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply:
- After 40 minutes of swimming or sweating
- Immediately after towel drying
- At least every two hours
- Children under 6 months of age:
Ask a doctor.
Sun Protection measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10am - 2pm
- Wear long-sleeved shirts, pants, hats and sunglasses
-
Inactive Ingredients
Dimethicone, Cyclopentasiloxane, Polysilicone-11, Squalane, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone, HDI/Trimethylol Hexyllactone Crosspolymer, Methyl Methacrylate Crosspolymer, Triethoxysilylethyl Polydimethylsiloxyethyl Hexyl Dimethicone, Palmitoyl Hexapeptide-12, Palmitoyl Tripeptide-1, Dunaliella Salina Extract, Ectoin, Hydroxymethoxyphenyl Decanone, Tocopherol, Caprylic/Capric Triglyceride, Stearalkonium Hectorite, Disteardimonium Hectorite, Pentaerythrityl Tetraisostearate, Propylene Carbonate
- Other Information
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - Kit Carton
-
INGREDIENTS AND APPEARANCE
PROCEDURE ENHANCEMENT SYSTEM
zinc oxide kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70604-040 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70604-040-00 1 in 1 BAG 09/17/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PUMP 53.2 mL Part 2 1 BOTTLE, PUMP 29.6 mL Part 3 1 TUBE 29.6 mL Part 4 1 TUBE 53.8 g Part 1 of 4 ULTRA CALM CLEANSING
cleansing (cold creams, cleansing lotions, liquids, and pads) [skin care preparations (creams, lotions, powder, and sprays)] creamProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR CETEARYL METHICONE (15000 MW) (UNII: VY9RTR7MSY) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) INGR POLYACRYLATE-13 (UNII: FS2D4T67EA) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) INGR POTASSIUM HYDROLYZED JOJOBA ESTERS (UNII: CH428W5O62) INGR PANTHENOL (UNII: WV9CM0O67Z) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR SOY STEROL (UNII: PL360EPO9J) INGR LINOLEIC ACID (UNII: 9KJL21T0QJ) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR GLYCERYL MONOOLEATE (UNII: C4YAD5F5G6) INGR STEARYL GLYCYRRHETINATE (UNII: 3YYE6VJS0P) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR BETAINE (UNII: 3SCV180C9W) INGR CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) INGR BENZYL ALCOHOL (UNII: LKG8494WBH) INGR TREMELLA FUCIFORMIS FRUITING BODY (UNII: GG8N28393G) INGR ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) INGR .GAMMA.-TOCOPHEROL (UNII: 8EF1Z1238F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 53.2 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 09/17/2021 Part 2 of 4 REGENERATING SKIN NECTAR
face and neck (excluding shaving preparations), leave-on [skin care preparations (creams, lotions, powder, and sprays)] lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) INGR PENTAERYTHRITYL TETRAISOSTEARATE (UNII: 9D7IK5483F) INGR HEPTYL UNDECYLENATE (UNII: W77QUB6GXO) INGR SOYBEAN OIL (UNII: 241ATL177A) INGR PANTHENOL TRIACETATE, (+)- (UNII: 1206E8961B) INGR SQUALANE (UNII: GW89575KF9) INGR CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR NARINGENIN (UNII: HN5425SBF2) INGR OLIVE OIL (UNII: 6UYK2W1W1E) INGR LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INGR PROPYLENE CARBONATE (UNII: 8D08K3S51E) INGR STEARALKONIUM HECTORITE (UNII: OLX698AH5P) INGR ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) INGR DUNALIELLA SALINA (UNII: F4O1DKI9A6) INGR .GAMMA.-TOCOPHEROL (UNII: 8EF1Z1238F) INGR PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) INGR PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) INGR ASCORBYL PALMITATE (UNII: QN83US2B0N) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 29.6 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 01/02/2017 Part 3 of 4 ULTRA LIGHT MOISTURIZER
moisturizing [skin care preparations (creams, lotions, powder, and sprays)] lotionProduct Information Route of Administration TOPICAL Other Ingredients Ingredient Kind Ingredient Name Quantity INGR WATER (UNII: 059QF0KO0R) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR DIMETHICONE (UNII: 92RU3N3Y1O) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) INGR POLYACRYLATE-13 (UNII: FS2D4T67EA) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR SQUALANE (UNII: GW89575KF9) INGR POLYISOBUTYLENE (1000 MW) (UNII: 5XB3A63Y52) INGR POTASSIUM HYDROLYZED JOJOBA ESTERS (UNII: CH428W5O62) INGR PANTHENOL (UNII: WV9CM0O67Z) INGR CAPRYLYL GLYCOL (UNII: 00YIU5438U) INGR XANTHAN GUM (UNII: TTV12P4NEE) INGR GLYCERYL MONOOLEATE (UNII: C4YAD5F5G6) INGR STEARYL GLYCYRRHETINATE (UNII: 3YYE6VJS0P) INGR CERAMIDE NP (UNII: 4370DF050B) INGR GREEN TEA LEAF (UNII: W2ZU1RY8B0) INGR EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) INGR .ALPHA.-BISABOLOL, (+/-)- (UNII: 36HQN158VC) INGR BETAINE (UNII: 3SCV180C9W) INGR ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) INGR CAPRYLHYDROXAMIC ACID (UNII: UPY805K99W) INGR POLYGLYCERYL-10 STEARATE (UNII: 90TF85HH91) INGR POLYSORBATE 20 (UNII: 7T1F30V5YH) INGR SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) INGR SUCROSE STEARATE (UNII: 274KW0O50M) INGR POTASSIUM SORBATE (UNII: 1VPU26JZZ4) INGR SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 29.6 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Cosmetic 09/17/2021 Part 4 of 4 SILKSHIELD ALL MINERAL SUNSCREEN WITH TRIHEX TECHNOLOGY
zinc oxide creamProduct Information Item Code (Source) NDC:70604-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.1995 g in 1 g Inactive Ingredients Ingredient Name Strength DIMETHICONE (UNII: 92RU3N3Y1O) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) SQUALANE (UNII: GW89575KF9) POLYGLYCERYL-3 POLYDIMETHYLSILOXYETHYL DIMETHICONE (4000 MPA.S) (UNII: RLA2U05Z4Q) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) TRIETHOXYSILYLETHYL POLYDIMETHYLSILOXYETHYL HEXYL DIMETHICONE (UNII: X75PL53TZJ) PALMITOYL HEXAPEPTIDE-12 (UNII: HO4ZT5S86C) PALMITOYL TRIPEPTIDE-1 (UNII: RV743D216M) DUNALIELLA SALINA (UNII: F4O1DKI9A6) ECTOINE (UNII: 7GXZ3858RY) 1-(4-HYDROXY-3-METHOXYPHENYL)-DECAN-3-ONE (UNII: BO24ID7E9U) TOCOPHEROL (UNII: R0ZB2556P8) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) STEARALKONIUM HECTORITE (UNII: OLX698AH5P) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PENTAERYTHRITYL TETRAISOSTEARATE (UNII: 9D7IK5483F) PROPYLENE CARBONATE (UNII: 8D08K3S51E) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70604-030-02 53.8 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/17/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 09/17/2021 Labeler - Alastin Skincare, Inc. (085997348)