Label: ERTACZO- sertaconazole nitrate cream
- NDC Code(s): 0187-5115-02, 0187-5115-60
- Packager: Bausch Health US, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated December 18, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use ERTACZO Cream safely and effectively. See full prescribing information for ERTACZO Cream.
ERTACZO® (sertaconazole nitrate) cream, for topical use
Initial U.S. Approval: 2003INDICATIONS AND USAGE
ERTACZO cream, 2% is an azole antifungal indicated for the topical treatment of interdigital tinea pedis in immunocompetent adult and pediatric patients 12 years of age and older caused by Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Cream, 2%. (3)
CONTRAINDICATIONS
None. (4)
ADVERSE REACTIONS
Most common adverse reactions observed in clinical trials (incidence >2%) were contact dermatitis, dry skin, burning skin, application site skin tenderness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch Health US, LLC at 1-800-321-4576 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Local Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
- •
- Apply ERTACZO cream, 2% twice daily for 4 weeks. Apply a sufficient amount of ERTACZO cream, 2% to cover both the affected areas between the toes and the immediately surrounding healthy skin.
- •
- Use ERTACZO cream, 2% for the full treatment time recommended by the physician, even though symptoms may have improved.
- •
- Dry the affected area(s) thoroughly before application, if using ERTACZO cream, 2% after bathing.
- •
- Wash hands after use.
- •
- Avoid the use of occlusive dressings or wrappings.
- •
- For topical use.
- •
- Not for ophthalmic, oral, or intravaginal use.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, cutaneous adverse events occurred in 7 of 297 (2%) subjects (2 of them severe) receiving ERTACZO cream, 2%, and in 7 of 291 (2%) subjects (2 of them severe) receiving vehicle. These reported cutaneous adverse events included contact dermatitis, dry skin, burning skin, and application site skin tenderness.
In a dermal sensitization trial, 8 of 202 evaluable subjects tested with ERTACZO cream, 2%, and 4 of 202 evaluable subjects tested with vehicle exhibited a erythematous reaction in the challenge phase. There was no evidence of cumulative irritation or contact sensitization in a repeated insult patch test involving 202 healthy volunteers.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ERTACZO cream, 2%. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cutaneous adverse events: erythema, pruritus, vesiculation, desquamation, and hyperpigmentation.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on ERTACZO cream, 2% use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, there were no adverse developmental effects observed with oral administration of sertaconazole nitrate to pregnant rats and rabbits during organogenesis at doses 40 and 80 times, respectively, the maximum recommended human dose (MRHD) based on body surface area (BSA) comparison. In rats, when maternal dosing was continued until weaning, a reduction in live birth indices and an increase in the number of still-born pups was observed at doses 20 and 40 times the MRHD based on BSA comparison (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of major birth defects, loss and other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
Animal embryofetal development studies have not been conducted with ERTACZO cream, 2%. Embryofetal development studies performed in pregnant rats and rabbits administered oral doses of sertaconzaole nitrate up to 160 mg/kg/day (40 times [rats] and 80 times [rabbits] the MRHD based on a BSA comparison) during the period of organogenesis revealed no malformations or embryofetal developmental toxicity. In a pre- and postnatal development study, pregnant rats were administered oral doses of sertaconazole nitrate from pregnancy day 6 to lactation day 20. A reduction in live birth indices and an increase in the number of still-born pups were seen at doses 20 and 40 times the MRHD based on BSA comparison.
8.2 Lactation
Risk Summary
There are no data available on the presence of sertaconazole in human or animal milk, its effects on the breastfed infant, or its effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ERTACZO cream, 2% and any potential adverse effects on the breastfed infant from ERTACZO cream, 2% or from the underlying maternal condition.
-
11 DESCRIPTION
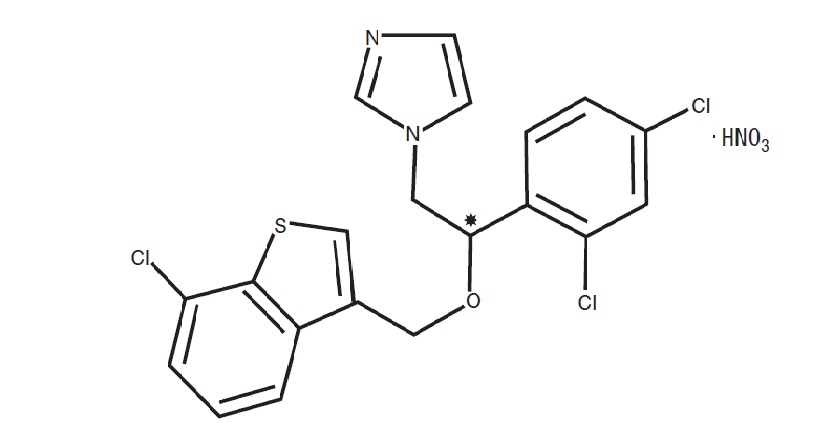
ERTACZO (sertaconazole nitrate) cream, 2%, is for topical application. It contains the azole antifungal, sertaconazole nitrate. Sertaconazole nitrate contains one asymmetric carbon atom and exists as a racemic mixture of equal amounts of R and S enantiomers.
Sertaconazole nitrate is designated chemically as (±)-1-[2,4-dichloro-β-[(7-chlorobenzo-[b]thien-3-yl)methoxy]phenethyl]imidazole nitrate. It has a molecular weight of 500.8. The molecular formula is C20H15Cl3N2OS ● HNO3, and the structural formula is as follows:
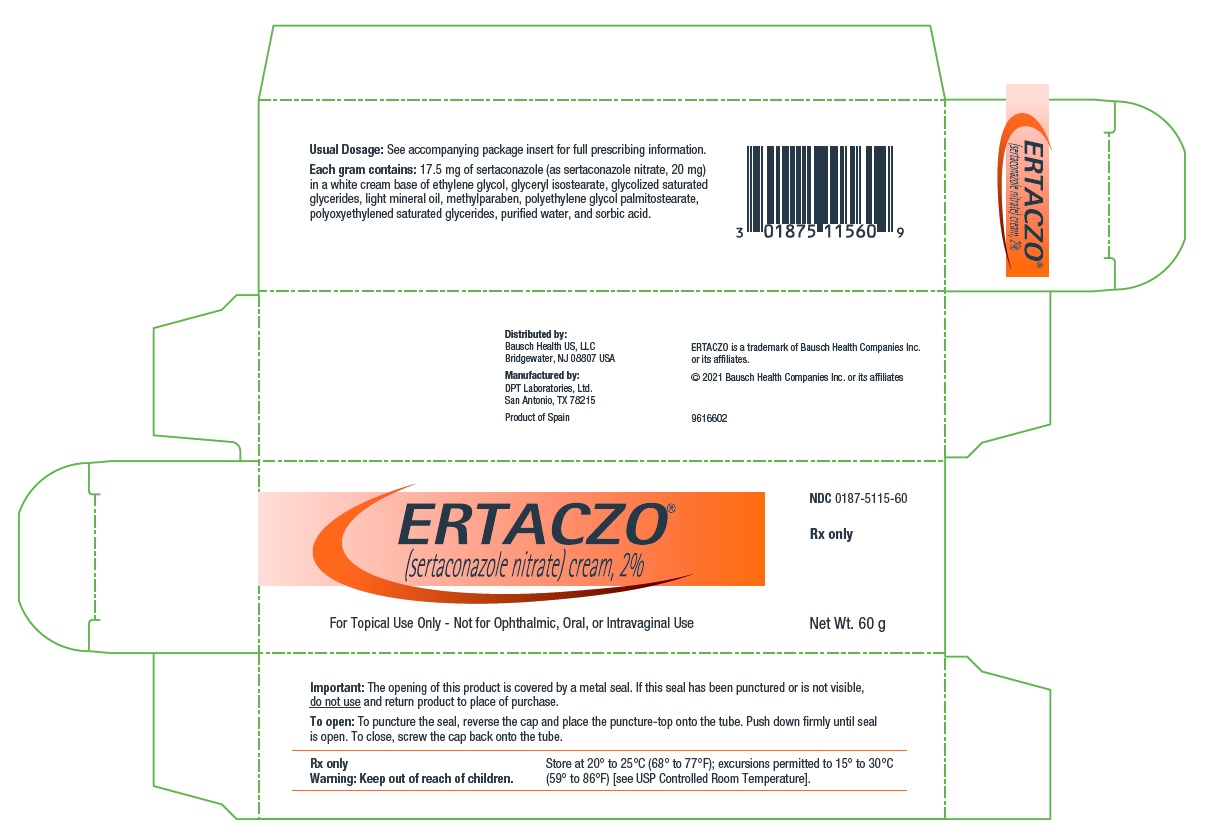
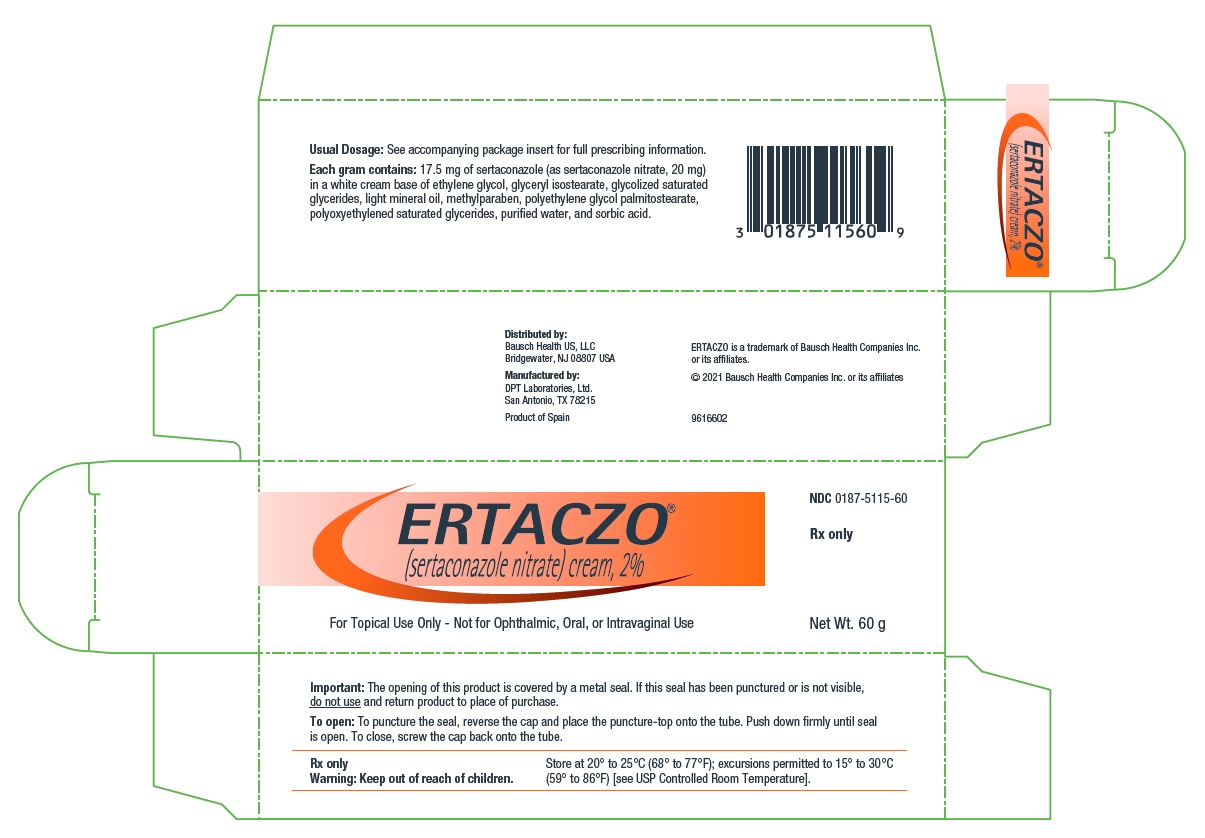
Sertaconazole nitrate is a white or almost white powder. It is practically insoluble in water, soluble in methanol, and sparingly soluble in alcohol and in methylene chloride. Each gram of ERTACZO cream, 2%, contains 17.5 mg of sertaconazole (as sertaconazole nitrate, 20 mg) in a white cream base of ethylene glycol, glyceryl isostearate, glycolized saturated glycerides, light mineral oil, methylparaben, polyethylene glycol palmitostearate, polyoxyethylened saturated glycerides, purified water, and sorbic acid.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Sertaconazole nitrate is an azole antifungal [see Clinical Pharmacology (12.4)].
12.3 Pharmacokinetics
In a multiple-dose pharmacokinetic trial that included 5 male subjects with interdigital tinea pedis (range of diseased area, 42 - 140 cm2; mean, 93 cm2), ERTACZO cream, 2%, was topically applied every 12 hours for a total of 13 doses to the diseased skin (0.5 g sertaconazole nitrate per 100 cm2). Sertaconazole concentrations in plasma measured by serial blood sampling for 72 hours after the thirteenth dose were below the limit of quantitation (2.5 ng/mL) of the analytical method used.
12.4 Microbiology
Mechanism of Action
Sertaconazole, an azole antifungal agent, inhibits fungal cytochrome P-450-mediated 14 alpha-lanosterol demethylase enzyme. This enzyme functions to convert lanosterol to ergosterol. Ergosterol is a key component of fungal cell membranes and lack of this component leads to fungal cell injury by leakage of key constituents in the cytoplasm from the cell.
Activity In Vitro and in Clinical Infections
Sertaconazole nitrate has been shown to be active against isolates of the following microorganisms in clinical infections [see Indications and Usage (1)]:
- Trichophyton rubrum
- Trichophyton mentagrophytes
- Epidermophyton floccosum
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 2-year rat dermal carcinogenicity study, topical administration of sertaconazole nitrate cream did not increase the number of neoplastic lesions compared to control animals, at sertaconazole nitrate doses of up to 800 mg/kg/day (200 times the MRHD based on a BSA comparison).
No clastogenic potential was observed in a mouse micronucleus test. Sertaconazole nitrate was nonclastogenic in the in vivo mouse sister chromatid exchange assay. There was no evidence that sertaconazole nitrate induced unscheduled DNA synthesis in primary rat hepatocyte cultures.
Rats treated orally with up to 160 mg/kg/day of sertaconazole nitrate (40 times the MRHD based on a BSA comparison), exhibited no toxicity or adverse effects on reproductive performance or fertility in male or female rats.
-
14 CLINICAL STUDIES
In two randomized, double-blind clinical trials, subjects 12 years and older with interdigital tinea pedis applied either ERTACZO cream, 2%, or vehicle, twice daily for 4 weeks. Subjects with moccasin-type (plantar) tinea pedis and/or onychomycosis were excluded from the trial. Two weeks after completion of therapy (6 weeks after beginning therapy), subjects were evaluated for signs and symptoms related to interdigital tinea pedis.
Treatment outcomes are summarized in the table below.
Treatment Outcomes as Percent (%) of Total Subjects with Interdigital Tinea Pedis Trial 1 Trial 2 Sertaconazole Vehicle Sertaconazole Vehicle Complete Cure* (Primary
Efficacy Variable)13/99
(13.1%)3/92
(3.3%)28/103
(27.2%)5/103
(4.9%)Effective Treatment**
32/99
(32.3%)11/92
(12.0%)52/103
(50.5%)16/103
(15.5%)Mycological Cure***
49/99
(49.5%)18/92
(19.6%)71/103
(68.9%)20/103
(19.4%)-
* Complete Cure – Patients who had complete clearing of signs and symptoms and Mycological Cure.
** Effective Treatment – Patients who had minimal residual signs and symptoms of interdigital tinea pedis and Mycological Cure.
*** Mycological Cure – Patients who had both negative microscopic KOH preparation and negative fungal culture.
- In clinical trials, complete cure in ERTACZO cream, 2%-treated subjects was achieved in 32 of 160 (20%) subjects with Trichophyton rubrum, in 7 of 28 (25%) subjects with Trichophyton mentagrophytes, and in 1 of 13 (15%) subjects with Epidermophyton floccosum.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Instruct the patient of the following:
Administration Instructions
- •
- ERTACZO cream, 2% is for topical use only.
- •
- Avoid contact with the eyes, mouth, vagina, and other mucous membranes.
- •
- Dry the affected area(s) thoroughly before application, if using ERTACZO cream, 2% after bathing.
- •
- Wash hands after use.
- •
- Use the medication for the full treatment time recommended by the physician, even though symptoms may have improved.
- •
- Avoid the use of occlusive dressings unless otherwise directed by the physician [see Dosage and Administration (2)].
Local Adverse Reactions
- •
- Inform the physician if the area of application shows signs of increased irritation, redness, itching, burning, blistering, swelling, or oozing [see Warnings and Precautions (5.1)].
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
DPT Laboratories, Ltd.
San Antonio, TX 78215
ERTACZO is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
9378102 -
Patient Package Insert
PATIENT INFORMATION
ERTACZO® (er-tack-zo)
(sertaconazole nitrate) cream
Important information: ERTACZO cream is for use on skin only. Do not use ERTACZO cream in your eyes, mouth, or vagina.
What is ERTACZO cream?
ERTACZO cream is a prescription medicine used on the skin (topical) to treat athlete’s foot that is between the toes (interdigital tinea pedis) in adults and children 12 years of age and older with normal immune systems.
It is not known if ERTACZO cream is safe and effective in children younger than 12 years of age.
Before using ERTACZO cream, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have any allergies
- •
- are pregnant or plan to become pregnant. It is not known if ERTACZO cream will harm your unborn baby.
- •
- are breastfeeding or plan to breastfeed. It is not known if ERTACZO cream passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you use ERTACZO cream.
Tell your healthcare provider about all of the medicinesyou take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use ERTACZO cream?
- •
- Use ERTACZO cream exactly as your healthcare provider tells you to use it.
- •
- Use ERTACZO cream for the full treatment time, even if your symptoms improve.
- •
- If you take a bath or shower, dry the affected skin areas well before you apply ERTACZO cream.
- •
- Apply ERTACZO cream 2 times a day for 4 weeks to the affected skin areas between your toes and to the healthy skin around the affected areas.
- •
- Wash your hands after you apply ERTACZO cream.
- •
- Do not cover the treated skin areas with bandages unless your healthcare provider tells you to.
What are the possible side effects of ERTACZO cream?
The most common side effects of ERTACZO cream include: redness, itching, dry skin, burning, blistering, swelling, drainage, and skin tenderness at the treated skin areas. Tell your healthcare provider if you have any of these skin reactions.
These are not all the possible side effects of ERTACZO cream.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ERTACZO cream?
- •
- Store ERTACZO cream at room temperature between 68°F to 77°F (20°C to 25°C).
- •
- Keep ERTACZO cream and all medicines out of reach of children.
General information about the safe and effective use of ERTACZO cream
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ERTACZO cream for a condition for which it was not prescribed. Do not give ERTACZO cream to other people, even if they have the same symptoms you have. It may harm them. You can ask your healthcare provider or pharmacist for information about ERTACZO cream that is written for health professionals.
What are the ingredients in ERTACZO cream?
Active ingredient: sertaconazole nitrate
Inactive ingredients: ethylene glycol, glyceryl isostearate, glycolized saturated glycerides, light mineral oil, methylparaben, polyethylene glycol palmitostearate, polyoxyethylened saturated glycerides, purified water, and sorbic acid
Distributed by:
Bausch Health US, LLC
Bridgewater, NJ 08807 USA
Manufactured by:
DPT Laboratories, Ltd.
San Antonio, TX 78215
ERTACZO is a trademark of Bausch Health Companies Inc. or its affiliates.
© 2020 Bausch Health Companies Inc. or its affiliates
9378102This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 12/2020 - PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 60 g Carton
-
INGREDIENTS AND APPEARANCE
ERTACZO
sertaconazole nitrate creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0187-5115 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sertaconazole Nitrate (UNII: 1DV05410M5) (Sertaconazole - UNII:72W71I16EG) Sertaconazole Nitrate 20 mg in 1 g Inactive Ingredients Ingredient Name Strength Ethylene Glycol (UNII: FC72KVT52F) Glyceryl Isostearate (UNII: HYE7O27HAO) Light Mineral Oil (UNII: N6K5787QVP) Methylparaben (UNII: A2I8C7HI9T) Sorbic Acid (UNII: X045WJ989B) PEG-6 STEARATE (UNII: 8LQC57C6B0) GLYCOL STEARATE (UNII: 0324G66D0E) PEG-32 STEARATE (UNII: 33GX5WQC0M) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0187-5115-60 1 in 1 CARTON 12/10/2003 1 60 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:0187-5115-02 18 in 1 CARTON 12/10/2003 11/30/2015 2 2 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021385 12/10/2003 Labeler - Bausch Health US, LLC (831922468) Establishment Name Address ID/FEI Business Operations DPT Laboratories, Ltd. 832224526 MANUFACTURE(0187-5115)