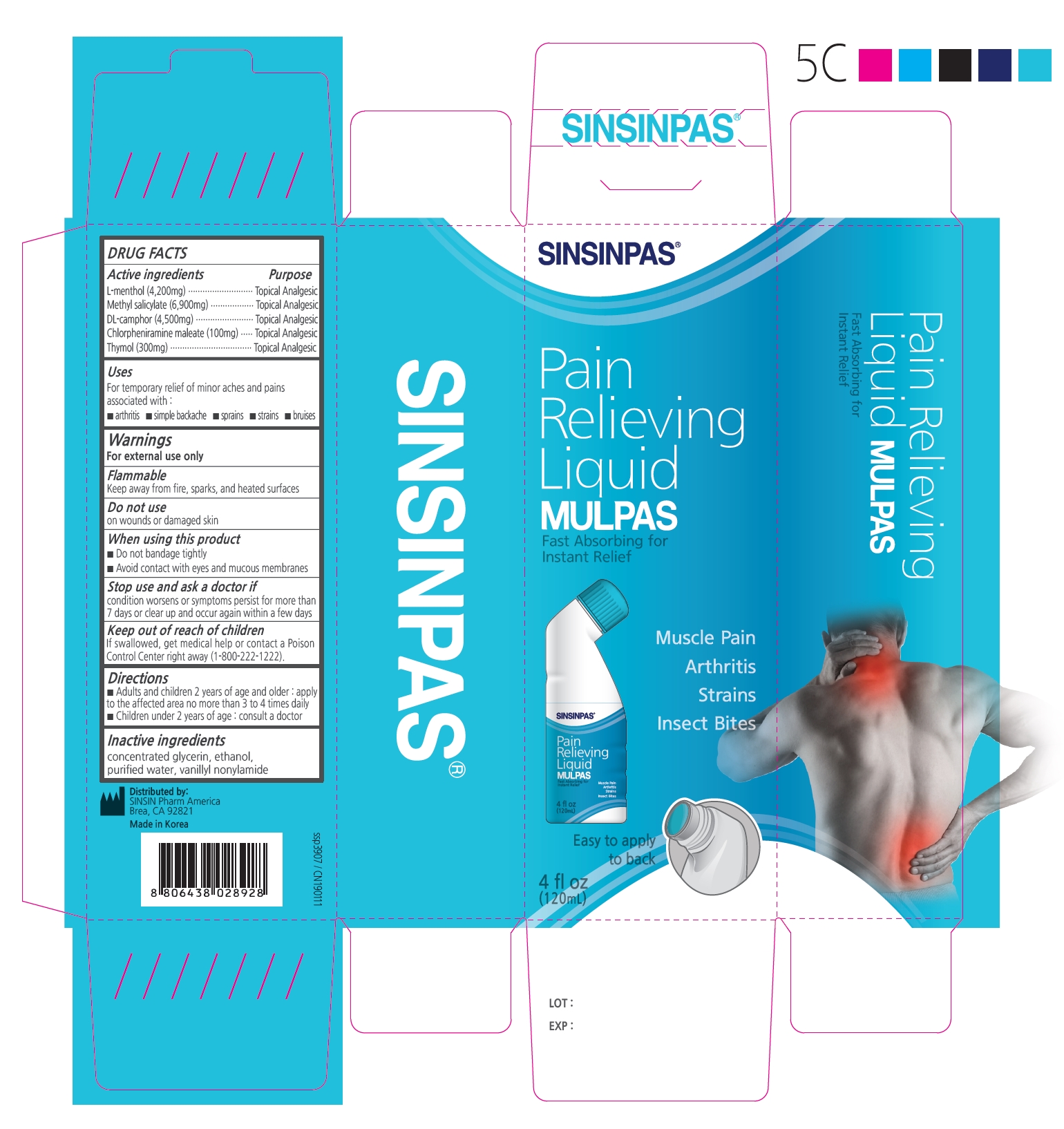
Label: SINSIN MULPAS- l-menthol, methyl salicylate, dl-camphor, chlorpheniramine maleate, thymol liquid
- NDC Code(s): 55264-107-01, 55264-107-02
- Packager: Sinsin Pharmaceutical Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Keep out of reach of children
- Uses
-
Warnings
For external use only
Do not use- on wounds or damaged skin
- if you are allergic to any ingredients of this product
- with a heating pad
- with, or at the same time as, other external analgesic products
When using this product
- do not use otherwise than directed
- avoid contact with eyes, mucous membranes or rashes
- do not bandage tightly
Ask a doctor before use if you are prone to allergic reaction from aspirin or salicylates
Stop use and ask a doctor if
- rash, itching, or excessive skin irritation develops
- conditions worsen
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
If pregnant or breast-feeding, ask a health professional before use.
- Directions
- Inactive Ingredients
- SINSIN MULPAS
-
INGREDIENTS AND APPEARANCE
SINSIN MULPAS
l-menthol, methyl salicylate, dl-camphor, chlorpheniramine maleate, thymol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:55264-107 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 4500 mg in 120 mL METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 6900 mg in 120 mL CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 100 mg in 120 mL MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 4200 mg in 120 mL Inactive Ingredients Ingredient Name Strength NONIVAMIDE (UNII: S846B891OR) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55264-107-02 1 in 1 BOX 02/12/2019 1 NDC:55264-107-01 120 mL in 1 CONTAINER; Type 0: Not a Combination Product 
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 02/01/2019 Labeler - Sinsin Pharmaceutical Co., Ltd. (823149161) Registrant - Sinsin Pharmaceutical Co., Ltd. (687867143) Establishment Name Address ID/FEI Business Operations Sinsin Pharmaceutical Co., Ltd. 687867143 manufacture(55264-107)