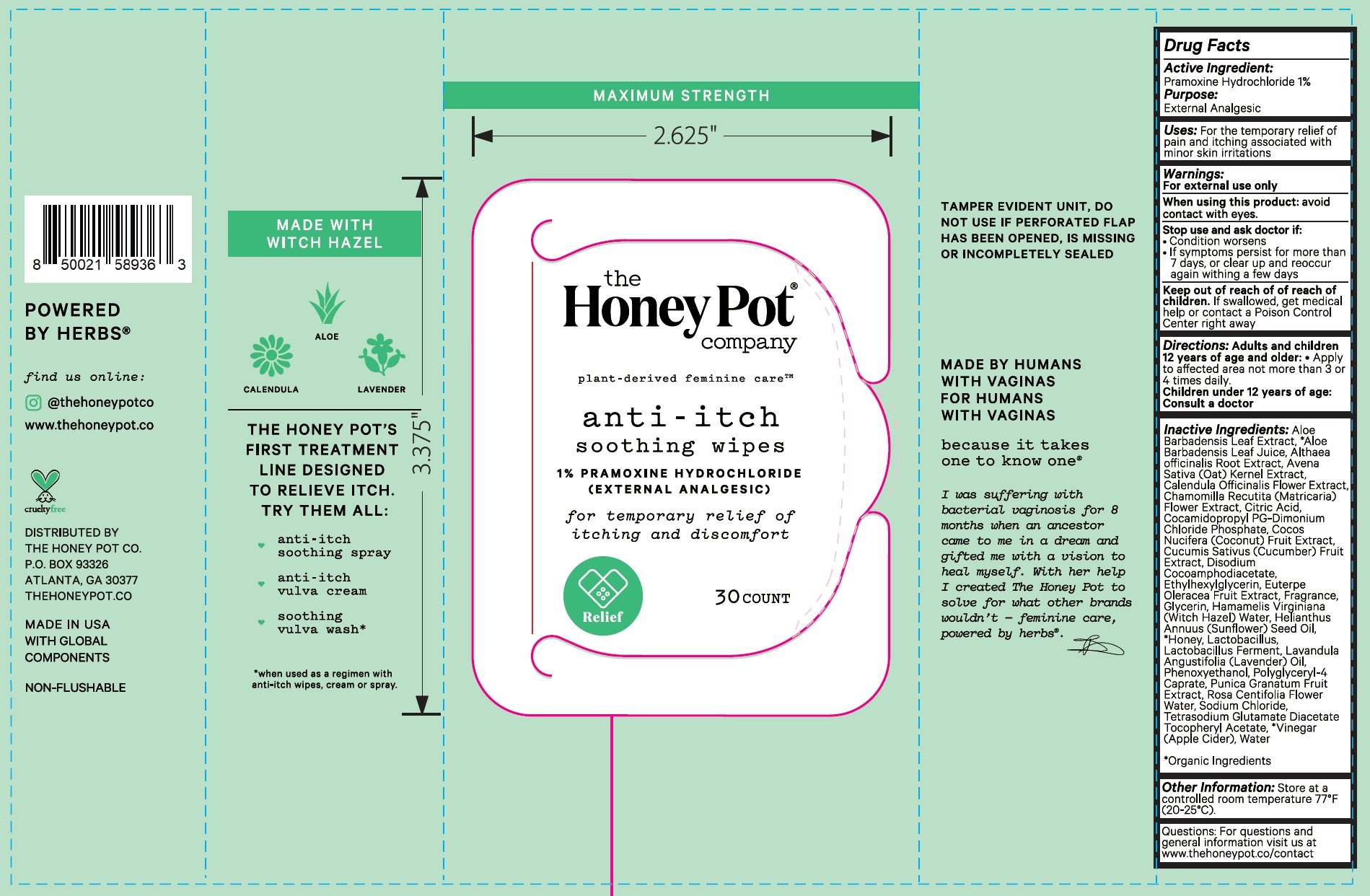
Label: ANTI-ITCH SOOTHING WIPES 1 PRAMOXINE HYDROCHLORIDE- pramoxine hydrochloride cloth
- NDC Code(s): 82637-9363-1
- Packager: The Honey Pot Company LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient:
- Uses:
- Warnings:
- Directions:
-
Inactive Ingredients:
Aloe Barbadensis Leaf Extract, *Aloe Barbadensis Leaf Juice, Althaea officinalis Root Extract, Avena Sativa (Oat) Kernel Extract, Calendula Officinalis Flower Extract, Chamomilla Recutita (Matricaria) Flower Extract, Citric Acid, Cocamidopropyl PG-Dimonium Chloride Phosphate, Cocos Nucifera (Coconut) Fruit Extract, Cucumis Sativus (Cucumber) Fruit Extract, Disodium Cocoamphodiacetate, Ethylhexylglycerin, Euterpe Oleracea Fruit Extract, Fragrance, Glycerin, Hamamelis Virginiana (Witch Hazel) Water, Helianthus Annuus (Sunflower) Seed Oil, *Honey, Lactobacillus, Lactobacillus Ferment, Lavandula Angustifolia (Lavender) Oil, Phenoxyethanol, Polyglyceryl-4 Caprate, Punica Granatum Fruit Extract, Rosa Centifolia Flower Water, Sodium Chloride, Tetrasodium Glutamate Diacetate Tocopheryl Acetate, *Vinegar (Apple Cider), Water *Organic Ingredients
- Other Information:
- Questions:
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
ANTI-ITCH SOOTHING WIPES 1 PRAMOXINE HYDROCHLORIDE
pramoxine hydrochloride clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82637-9363 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) ALTHAEA OFFICINALIS ROOT (UNII: TRW2FUF47H) OAT (UNII: Z6J799EAJK) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CHAMOMILE (UNII: FGL3685T2X) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) COCAMIDOPROPYL PROPYLENE GLYCOL-DIMONIUM CHLORIDE PHOSPHATE (UNII: H2KVQ74JM4) COCONUT (UNII: 3RT3536DHY) CUCUMBER (UNII: YY7C30VXJT) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ACAI (UNII: 46AM2VJ0AW) GLYCERIN (UNII: PDC6A3C0OX) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) SUNFLOWER OIL (UNII: 3W1JG795YI) HONEY (UNII: Y9H1V576FH) LAVENDER OIL (UNII: ZBP1YXW0H8) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYGLYCERYL-4 CAPRATE (UNII: 3N873UN885) POMEGRANATE (UNII: 56687D1Z4D) ROSA CENTIFOLIA FLOWER OIL (UNII: H32V31VMWY) SODIUM CHLORIDE (UNII: 451W47IQ8X) TETRASODIUM GLUTAMATE DIACETATE (UNII: 5EHL50I4MY) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ACETIC ACID (UNII: Q40Q9N063P) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82637-9363-1 30 in 1 POUCH 03/01/2022 1 2.8 mL in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 03/01/2022 Labeler - The Honey Pot Company LLC (045600502)