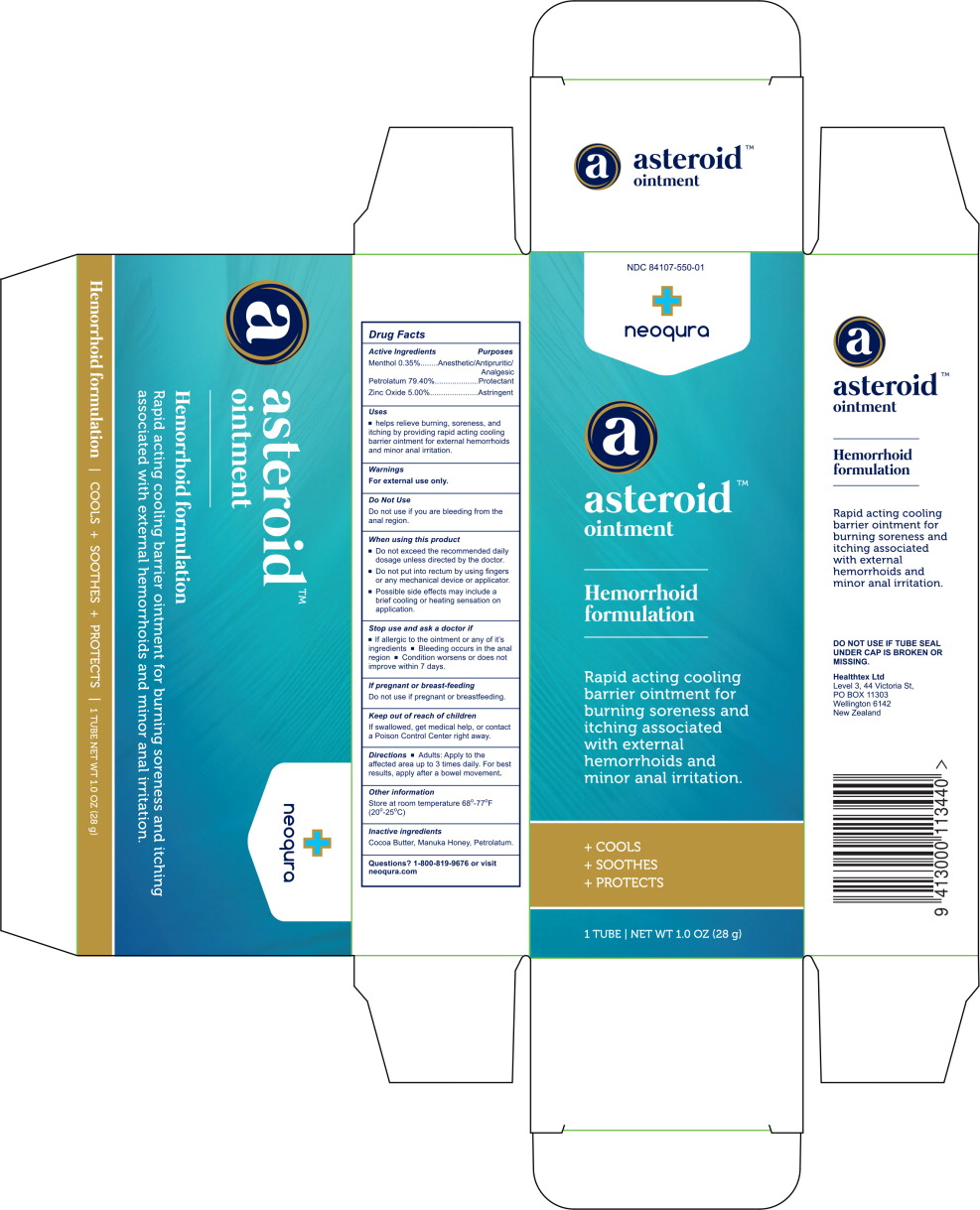
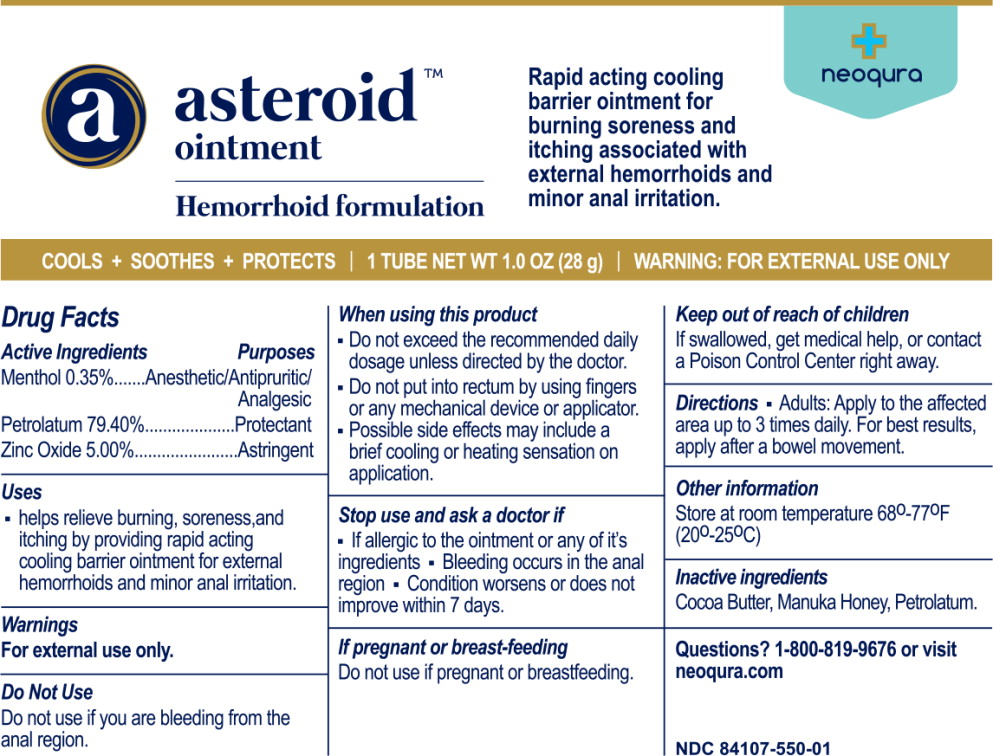
Label: ASTEROID- menthol, petrolatum, zinc oxide ointment
- NDC Code(s): 84107-550-01
- Packager: HEALTHTEX LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredients
Menthol 0.35% Petrolatum 79.40% Zinc Oxide 5.00%
-
Purposes
Anesthetic/Antipruritic/Analgesic - Protectant - Astringent
-
Uses
helps relieve burning, soreness, and itching by providing rapid acting cooling barrier ointment for external hemorrhoids and minor anal irritation.
-
Warnings
For external use only. Do Not Use - Do not use if you are bleeding from the anal region. When using this product - Do not exceed the recommended daily dosage unless directed by the ...
-
Directions
Adults: Apply to the affected area up to 3 times daily. For best results, apply after a bowel movement.
-
Other information
Store at room temperature 68°-77°F (20°-25°C)
-
Inactive ingredients
Cocoa Butter, Manuka Honey, Petrolatum.
-
Questions?
1-800-819-9676 or visit neoqura.com
-
Principal Display Panel – 28 g Carton Label
NDC 84107-550-01 - neoqura - asteroid™ ointment - Hemorrhoid - formulation - Rapid acting cooling - barrier ointment for - burning soreness and - itching associated - with external - hemorrhoids and - minor anal ...
-
Principal Display Panel – 28 g Tube Label
neoqura - asteroid™ ointment - Hemorrhoid formulation - Rapid acting cooling - barrier ointment for - burning soreness and - itching associated with - external hemorrhoids and - minor anal ...
-
INGREDIENTS AND APPEARANCEProduct Information