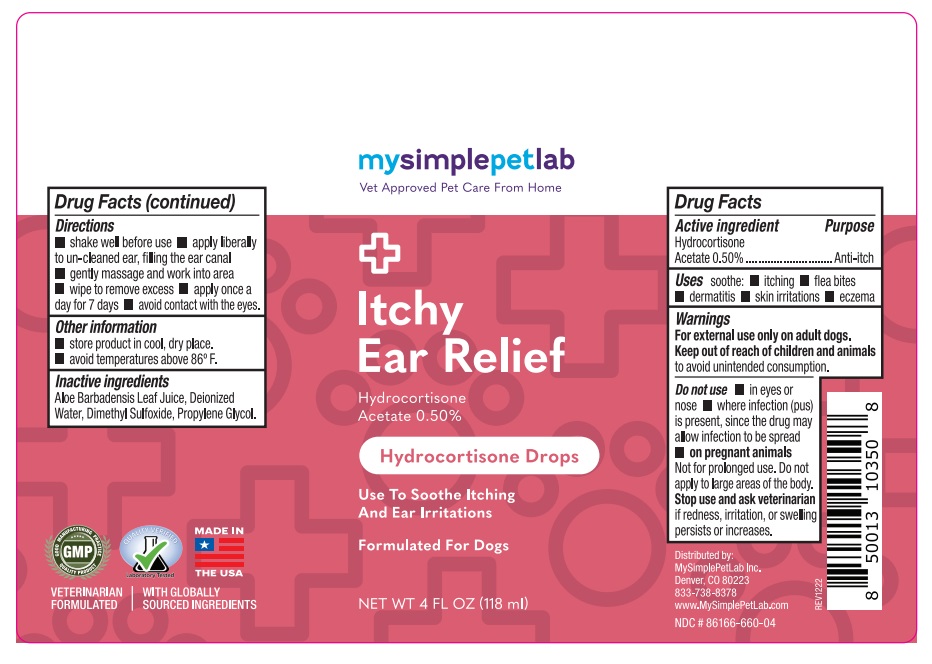
Label: MYSIMPLEPETLAB ITCHY EAR RELIEF- hydrocortisone acetate solution/ drops
- NDC Code(s): 86166-660-04
- Packager: Mysimplepetlab Inc
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated March 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use on adult dogs.
Keep out of reach of children and animals to avoid unintended consumption.Do not use • in eyes or nose • where infection (pus) is present, since the drug may allow infection to be spread
• on pregnant animals
Not for prolonged use. Do not apply to large areas of the body.
Stop use and ask veterinarian if redness, irritation, or swelling persists or increases. - Directions
- Other information
- Inactive ingredients
- SPL UNCLASSIFIED SECTION
- Packaging
-
INGREDIENTS AND APPEARANCE
MYSIMPLEPETLAB ITCHY EAR RELIEF
hydrocortisone acetate solution/ dropsProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86166-660 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE ACETATE (UNII: 3X7931PO74) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE ACETATE 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) DIMETHYL SULFOXIDE (UNII: YOW8V9698H) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86166-660-04 118 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2023 Labeler - Mysimplepetlab Inc (118813078)