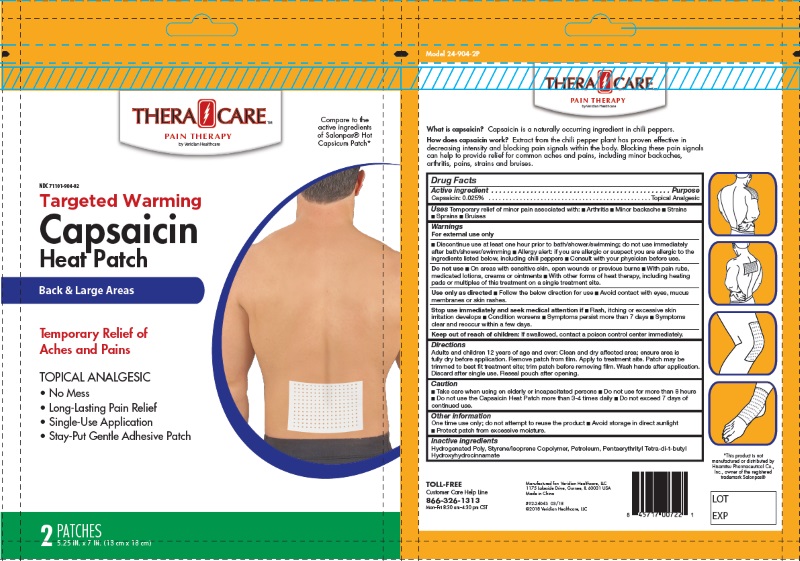
Label: 2-COUNT HEAT PATCHES- capsaicin patch
- NDC Code(s): 71101-904-02
- Packager: Veridian Healthcare
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 21, 2018
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- For External Use Only
- Uses
- Purpose
- Active Ingredients
- Inactive Ingredients:
-
Directions for Use:
For Use on Adults and Childrens 12 Years of Age or Older
- Clean and dru affected area; ensure area is fully dry before application
- Remove patch from film
- Apply to treatement site
- Patch may be trimmerd to best fit treatment site; trim patch before removing film
- Wash hands after application
- Discard after signle use
- Take care when using on elderly ot incapacited persons
- Do not use more than 8 hours
- Do not use the Capsaicin Heat Patch more than 3-4 times daily
- Do not exceed 7 days of continue use
- Keep Out of Reach of Children
- Storage and Care:
-
Do Not Use:
- On areas with sensitive skin, open wounds or previous burns
- with pain rubs, medicated lotions, creams or oinments
- with other forms of heat theraphy, including heating pads, or multiples of this treatment on a single treatement site
- QUESTIONS
- LABEL
-
INGREDIENTS AND APPEARANCE
2-COUNT HEAT PATCHES
capsaicin patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71101-904 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAPSAICIN (UNII: S07O44R1ZM) (CAPSAICIN - UNII:S07O44R1ZM) CAPSAICIN 0.25 g in 1 g Inactive Ingredients Ingredient Name Strength HYDROGENATED POLYDECENE (550 MW) (UNII: U333RI6EB7) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) LIQUID PETROLEUM (UNII: 6ZAE7X688J) STYRENE/ACRYLAMIDE COPOLYMER (MW 500000) (UNII: 5Z4DPO246A) Product Characteristics Color Score Shape RECTANGLE Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71101-904-02 2 in 1 BOX 11/21/2018 1 9 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 11/21/2018 Labeler - Veridian Healthcare (830437997) Establishment Name Address ID/FEI Business Operations Foshan Aqua Gel Biotech Co.,Ltd. 529128763 manufacture(71101-904)