Label: VETERICYN TRIPLE ACTION EAR TREATMENT- hydrocortisone liquid
- NDC Code(s): 70489-1023-1
- Packager: INNOVACYN INC.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Description
- Dosage/Administration
-
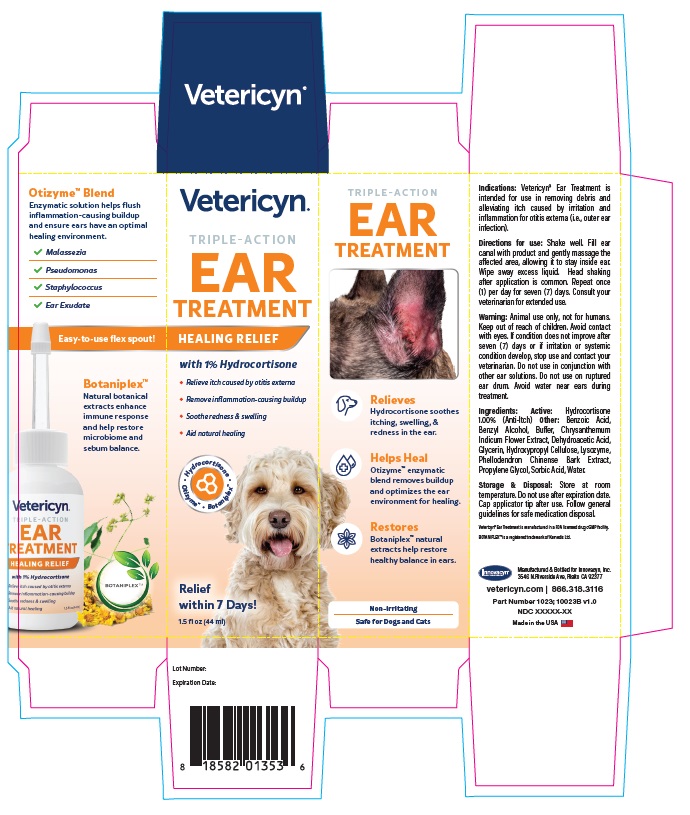
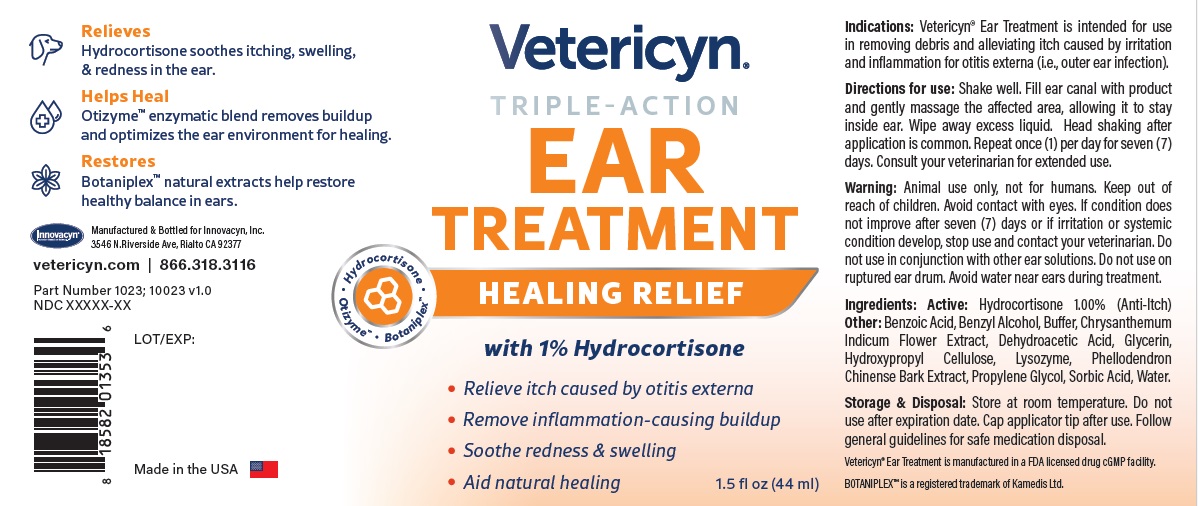
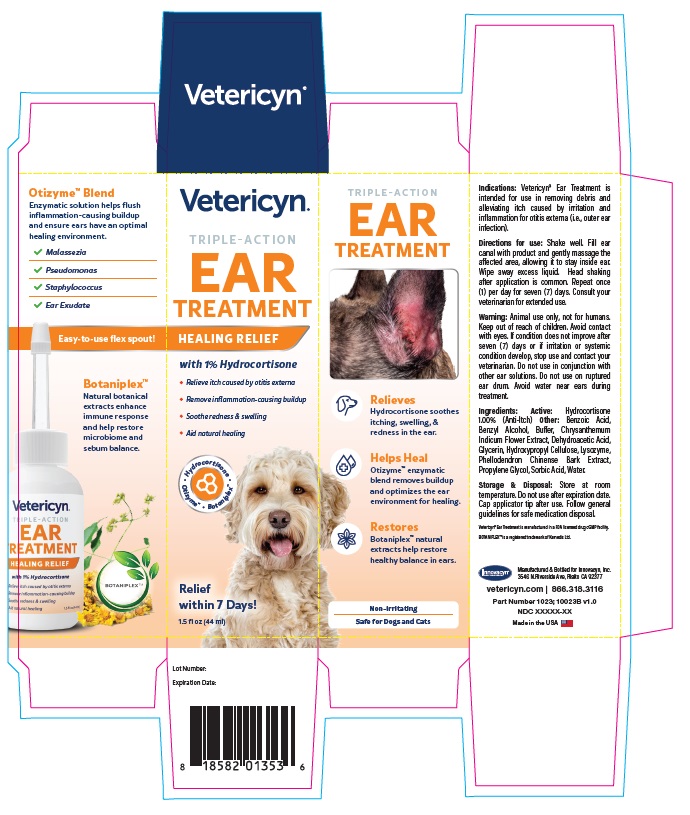
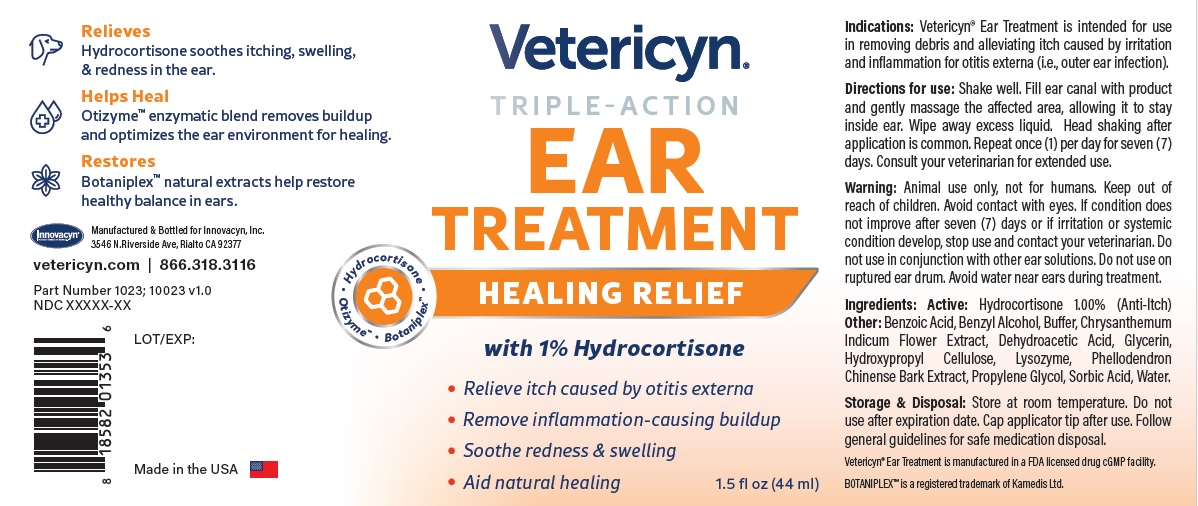
Warning
Animal use only, not for humans. Keep out of reach of children. Avoid contact with eyes. If condition does not improve after seven (7) days or if irritation or systemic condition develop, stop use and contact your veterinarian. Do not use in conjunction with other ear solutions. Do not use on ruptured ear drum. Avoid water near ears during treatment.
- Inactive Ingredient
- Precautions
- Questions
- Product label
-
INGREDIENTS AND APPEARANCE
VETERICYN TRIPLE ACTION EAR TREATMENT
hydrocortisone liquidProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:70489-1023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) BENZYL ALCOHOL (UNII: LKG8494WBH) BENZOIC ACID (UNII: 8SKN0B0MIM) SORBIC ACID (UNII: X045WJ989B) DEHYDROACETIC ACID (UNII: 2KAG279R6R) CHRYSANTHEMUM INDICUM FLOWER (UNII: I6OER6U04L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) WATER (UNII: 059QF0KO0R) LYSOZYME (UNII: 968JKA7T33) SODIUM HYDROXIDE (UNII: 55X04QC32I) PHELLODENDRON CHINENSE WHOLE (UNII: QKA3ZK8IIE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70489-1023-1 1 in 1 CARTON 1 44 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/01/2024 Labeler - INNOVACYN INC. (025217003) Establishment Name Address ID/FEI Business Operations Westwood Laboratories, LLC. 832280635 api manufacture