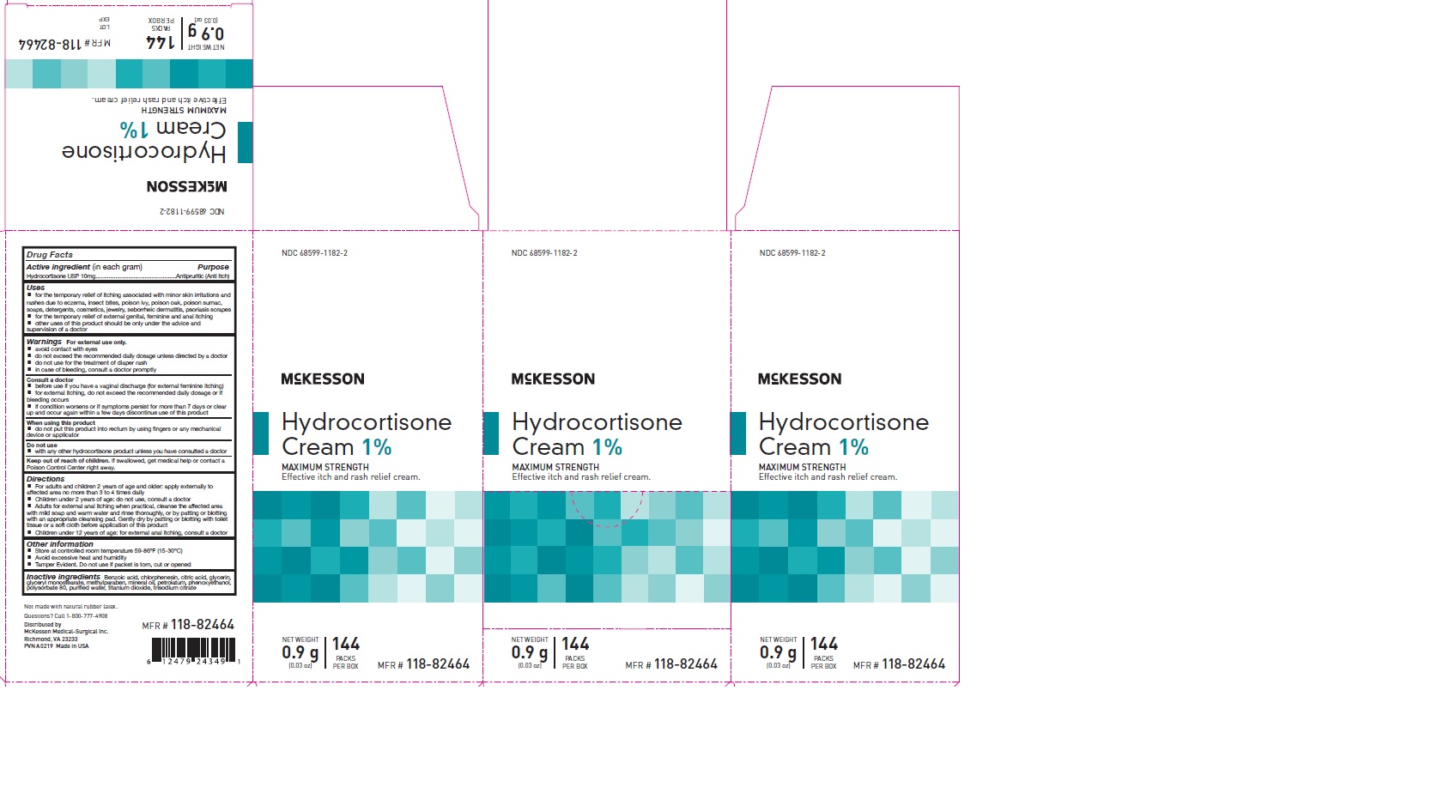
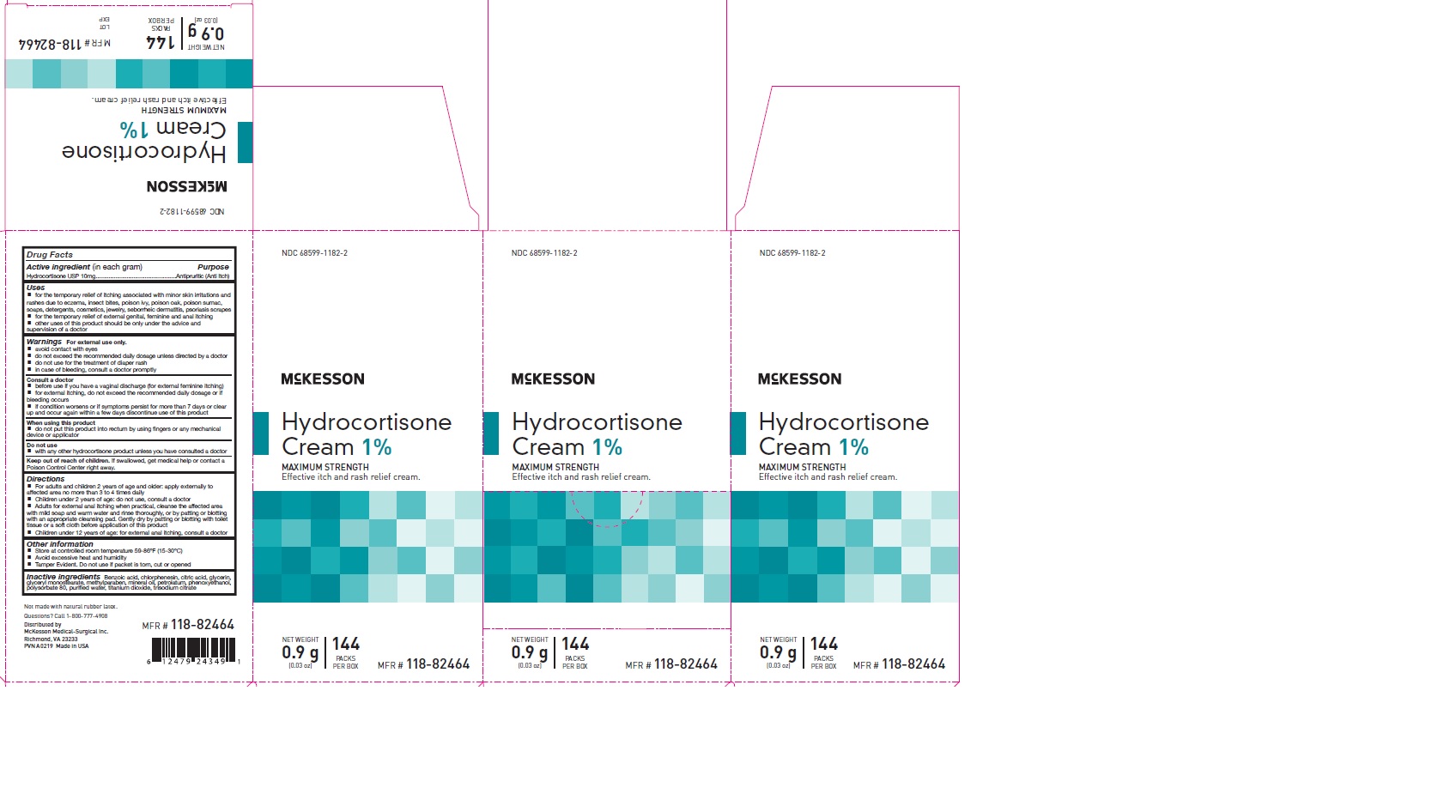
Label: HYDROCORTISONE- hydrocortisone cream cream
- NDC Code(s): 68599-1182-2
- Packager: McKesson
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Indications:
For the temporary relief of itching associated with minor skin irritation and rashes due to eczema, insect bites, poison ivy, poison oak, poison sumac, soaps, detergents, cosmetics, jewelry, seborrheic dermatitis, psoriasis scrapes
For the temporary relief of external genital, feminine and anal itching
Other uses of this product should be only under the advice and supervision of a doctor -
WARNINGS
Warnings:
For external use only- Avoid contact with eyes
- Do not exceed the recommended daily dosage unless directed by a doctor
- Do no use for treatment of diaper rash
- In case of bleeding, consult a doctor promptly
Consult a doctor:
- Before use if you have a vaginal discharge (for external feminine itching)
- For external itching, do not exceed the recommended daily dosage or if bleeding occurs
- If condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days discontinue use of this product
When using this product:
- Do not put this product into rectum by using fingers or any mechanical device or applicator
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions:
- For adults and children 2 years of age and older: apply externally to affected area not more than 3 to 4 times daily.
- Children under 2 years of age: do not use, consult a doctor
- Adults for external anal itching when practical, cleanse the affected area with mild soap and warm water and rinse thoroughly, or by patting or blotting with appropriate cleansing pad. Gently dry by patting or blotting with toilet tissue or a soft cloth before application of this product.
- Children under 12 years or age; for external anal itching, consult a doctor.
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HYDROCORTISONE
hydrocortisone cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68599-1182 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 100 g Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) POLYSORBATE 80 (UNII: 6OZP39ZG8H) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) METHYLPARABEN (UNII: A2I8C7HI9T) MINERAL OIL (UNII: T5L8T28FGP) PHENOXYETHANOL (UNII: HIE492ZZ3T) BENZOIC ACID (UNII: 8SKN0B0MIM) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CHLORPHENESIN (UNII: I670DAL4SZ) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68599-1182-2 0.9 g in 1 PACKET; Type 0: Not a Combination Product 03/14/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 03/13/2019 07/01/2024 Labeler - McKesson (023904428) Establishment Name Address ID/FEI Business Operations Ultra Seal Corporation 085752004 pack(68599-1182)