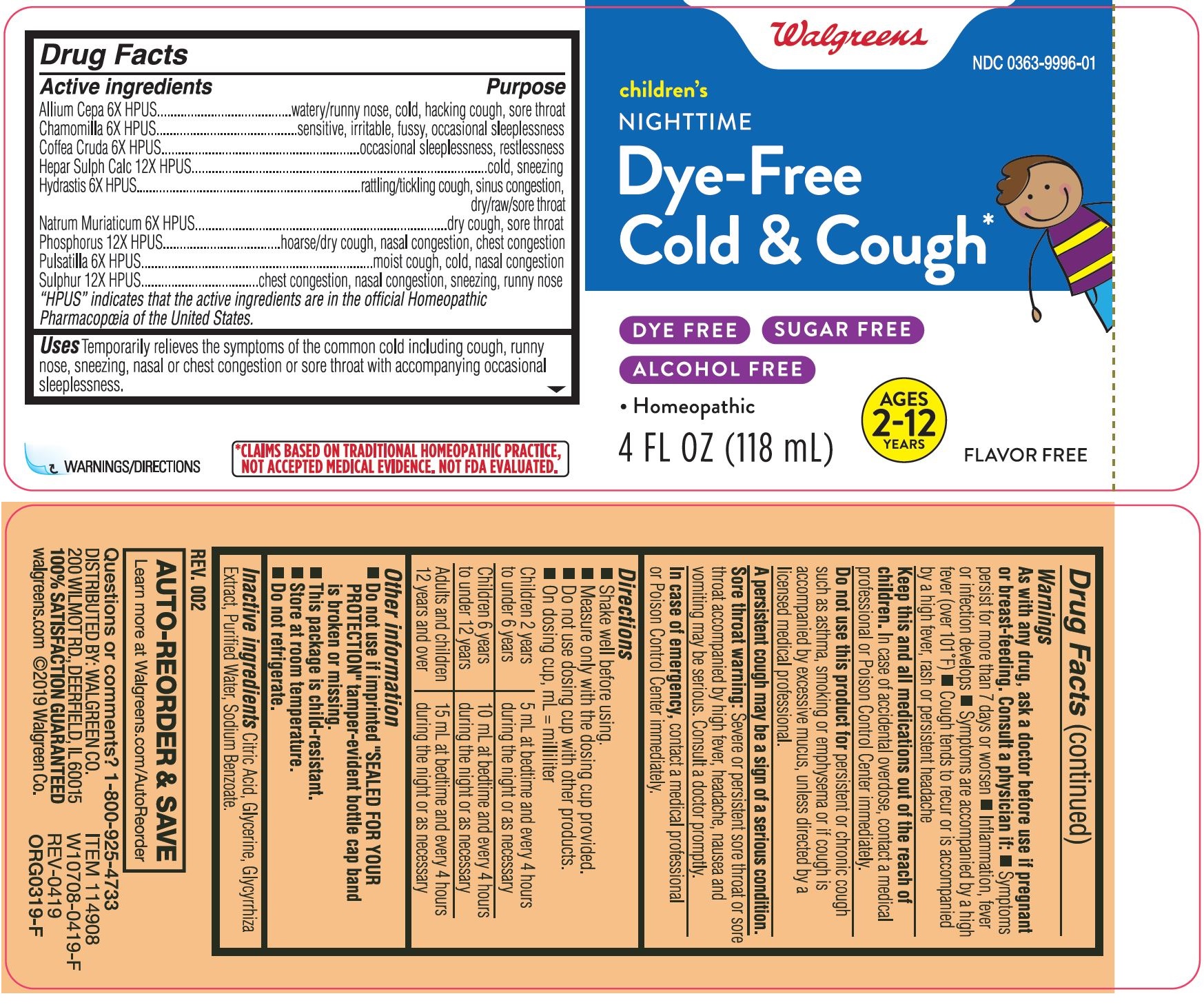
Label: CHILDRENS COLD AND COUGH NIGHTTIME- onion, calcium sulfide, sodium chloride, anemone pulsatilla, arabica coffee bean, matricaria chamomilla, sulfur, phosphorus and goldenseal liquid
- NDC Code(s): 0363-9996-01
- Packager: Walgreen Company
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 15, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Drug Facts
Active ingredients
Allium Cepa 6X HPUS
Chamomilla 6X HPUS
Coffea Cruda 6X HPUS
Hepar Sulph Calc 12X HPUS
Hydrastis 6X HPUS
Natrum Muriaticum 6X HPUS
Phosphorus 12X HPUS
Pulsatilla 6X HPUS
Sulphur 12X HPUS
“HPUS” indicates that the active ingredients are in the official Homeopathic
Pharmacopœia of the United States.
Purpose
Allium Cepa 6X HPUS...................watery/runny nose, cold, hacking cough, sore throat
Chamomilla 6X HPUS...................sensitive, irritable, fussy, occasional sleeplessness
Coffea Cruda 6X HPUS.................occasional sleeplessness, restlessness
Hepar Sulph Calc 12X HPUS........cold, sneezing
Hydrastis 6X HPUS.......................rattling/tickling cough, sinus congestion, dry/raw/sore throat
Natrum Muriaticum 6X HPUS.......dry cough, sore throat
Phosphorus 12X HPUS................hoarse/dry cough, nasal congestion, chest congestion
Pulsatilla 6X HPUS.......................moist cough, cold, nasal congestion
Sulphur 12X HPUS.......................chest congestion, nasal congestion, sneezing, runny nose
- Uses
-
Drug Facts (continued)
Warnings
Keep this and all medications out of the reach of children.
In case of accidental overdose, contact a medical professional or Poison Control Center immediately.
Do not use this product
for persistent or chronic cough such as asthma, smoking or emphysema or if cough is accompanied by excessive mucus, unless directed by a licensed medical professional.
-
Directions
- Shake well before using.
- Measure only with the dosing cup provided.
- Do not use dosing cup with other products.
- On dosing cup, mL = milliliter.
Children 2 years
to under 6 years
5 mL at bedtime and every 4 hours
during the night or as necessary
Children 6 years
to under 12 years
10 mL at bedtime and every 4 hours
during the night or as necessary
Adults and children
12 years and over
15 mL at bedtime and every 4 hours
during the night or as necessary
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- Principal Display Panel - 118 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
CHILDRENS COLD AND COUGH NIGHTTIME
onion, calcium sulfide, sodium chloride, anemone pulsatilla, arabica coffee bean, matricaria chamomilla, sulfur, phosphorus and goldenseal liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0363-9996 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 6 [hp_X] in 1 mL ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 6 [hp_X] in 1 mL MATRICARIA CHAMOMILLA (UNII: G0R4UBI2ZZ) (MATRICARIA CHAMOMILLA - UNII:G0R4UBI2ZZ) MATRICARIA CHAMOMILLA 6 [hp_X] in 1 mL GOLDENSEAL (UNII: ZW3Z11D0JV) (GOLDENSEAL - UNII:ZW3Z11D0JV) GOLDENSEAL 6 [hp_X] in 1 mL ARABICA COFFEE BEAN (UNII: 3SW678MX72) (ARABICA COFFEE BEAN - UNII:3SW678MX72) ARABICA COFFEE BEAN 6 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 1 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 12 [hp_X] in 1 mL ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 6 [hp_X] in 1 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) GLYCERIN (UNII: PDC6A3C0OX) SODIUM BENZOATE (UNII: OJ245FE5EU) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0363-9996-01 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/01/2019 Labeler - Walgreen Company (008965063) Establishment Name Address ID/FEI Business Operations Hyland's Inc. 008316655 pack(0363-9996) , manufacture(0363-9996) , label(0363-9996)