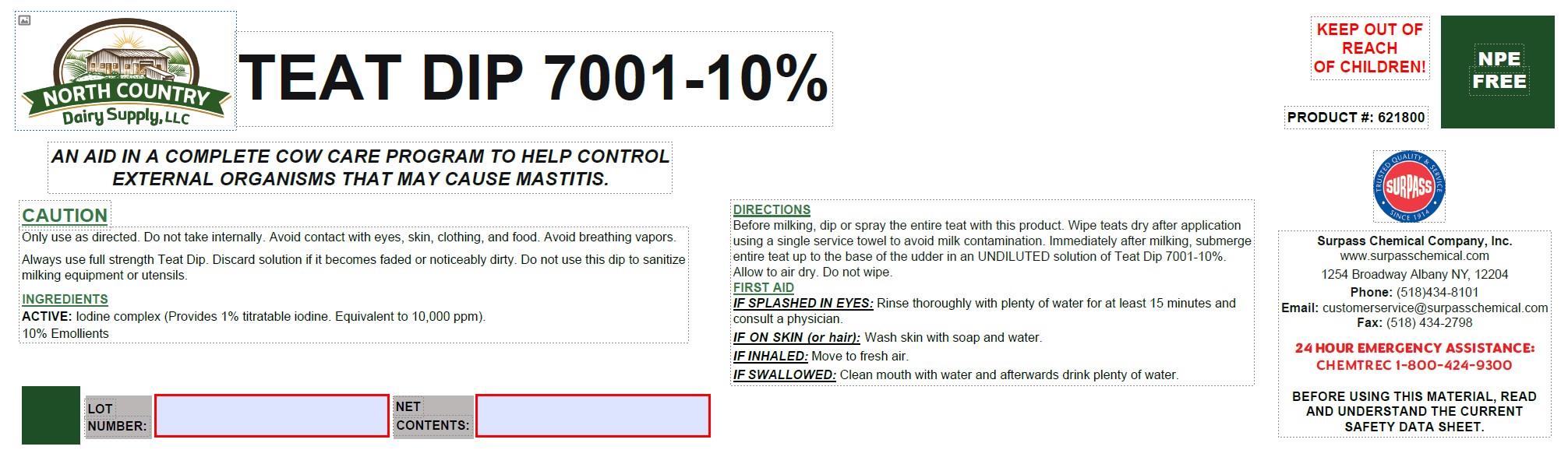
Label: TEAT DIP 7001-10%- iodine solution
- NDC Code(s): 86067-0112-3, 86067-0112-4, 86067-0112-6, 86067-0112-7
- Packager: Surpass Chemical Company, Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated May 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DESCRIPTION
- INSTRUCTIONS FOR USE
- GENERAL PRECAUTIONS
- ACTIVE INGREDIENT
-
OTHER SAFETY INFORMATION
FIRST AID:
IF SPLASHED IN EYES: Rinse immediately with plenty of water, also under the eyelids, for at least 15 minutes. Immediate medical attention is required.
IF ON SKIN (or hair): Wash off immediately with soap and plenty of water removing all contaminated clothes and shoes. Immediate medical attention is required.
IF INHALED: Move to fresh air. If breathing is difficult, give oxygen. Immediate medical attention is required.
IF SWALLOWED: Drink plenty of water. Never give anything by mouth to an unconscious person. Consult a physician if necessary. - SAFE HANDLING WARNING
- SPL UNCLASSIFIED SECTION
- KEEP OUT OF REACH OF CHILDREN
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TEAT DIP 7001-10%
iodine solutionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:86067-0112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IODINE (UNII: 9679TC07X4) (IODINE - UNII:9679TC07X4) IODINE 0.0472 kg in 1 kg Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) 0.794 kg in 1 kg C9-11 PARETH-8 (UNII: 80E6PSE1XL) 0.051 kg in 1 kg GLYCERIN (UNII: PDC6A3C0OX) 0.0984 kg in 1 kg TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) 0.007 kg in 1 kg XANTHAN GUM (UNII: TTV12P4NEE) 0.008 kg in 1 kg 1,3-BUTYLENE GLYCOL DIOLEATE (UNII: 81H4CJ8388) 0.0016 kg in 1 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:86067-0112-3 20.41 kg in 1 PAIL 2 NDC:86067-0112-4 60.78 kg in 1 DRUM 3 NDC:86067-0112-6 222.71 kg in 1 DRUM 4 NDC:86067-0112-7 1294.55 kg in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/10/2023 Labeler - Surpass Chemical Company, Inc. (002075133) Establishment Name Address ID/FEI Business Operations Surpass Chemical Company, Inc. 002075133 manufacture, api manufacture