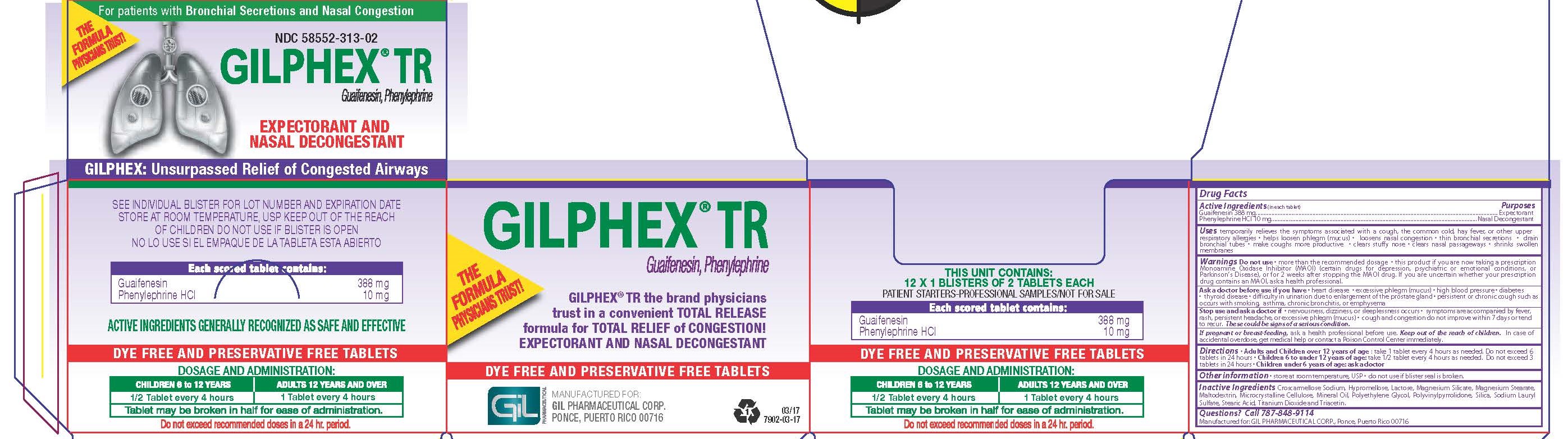
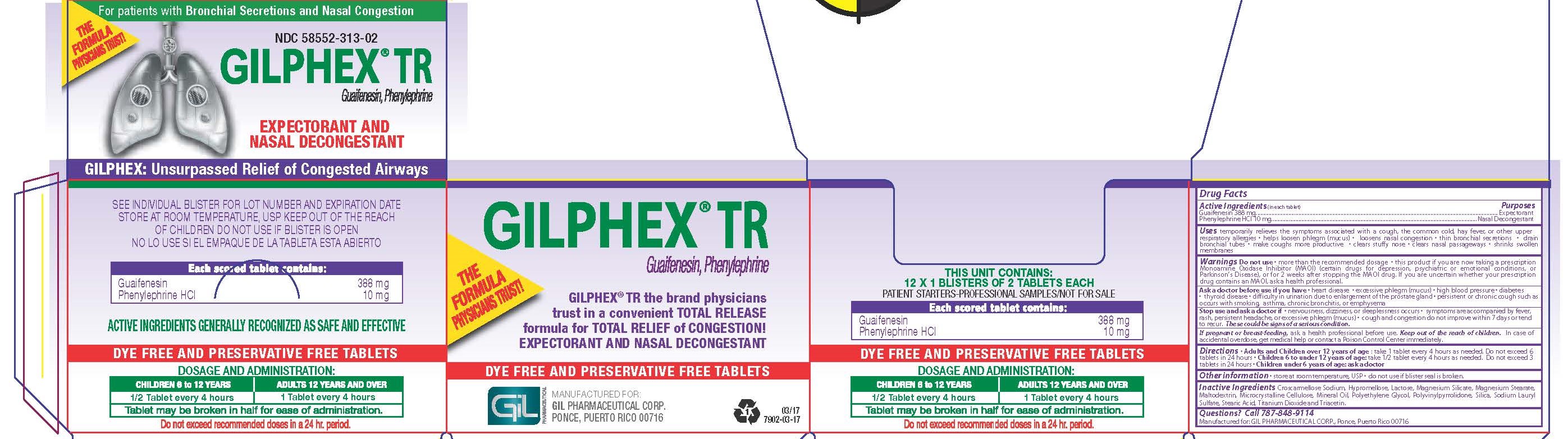
Label: GILPHEX TR- guaifenesin and phenylephrine hcl tablet, film coated
-
Contains inactivated NDC Code(s)
NDC Code(s): 58552-313-01, 58552-313-02 - Packager: Gil Pharmaceutical Corp
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 16, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- PURPOSE
-
Uses
temporarily relieves these symptoms associated with a cough, the common cold, hay fever, or other upper respiratory allergies
- helps loosen phlegm (mucus)
- loosens nasal congestion
- thin bronchial secretions
- drain bronchial tubes
- make coughs more productive
- clears stuffy nose
- clear nasal passageways
- shrinks swollen membranes
-
WARNINGS
Do not use this product more than the recommended dosage, or if you are now taking a prescription Monoamine Oxidase Inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s Disease), or for 2 weeks after stopping the MAOI drug. If you are uncertain whether your prescription drug contains an MAOI, ask a health professional.
Ask a doctor before use if you have
- heart disease
- excessive phlegm (mucus)
- high blood pressure
- diabetes
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- Directions
- Other information
- INACTIVE INGREDIENT
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GILPHEX TR
guaifenesin and phenylephrine hcl tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58552-313 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 388 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE (UNII: J2B2A4N98G) TALC (UNII: 7SEV7J4R1U) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) LIGHT MINERAL OIL (UNII: N6K5787QVP) POVIDONE K30 (UNII: U725QWY32X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color white Score 2 pieces Shape OVAL Size 19mm Flavor Imprint Code GIL;GIL;304;304 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58552-313-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 05/13/2011 2 NDC:58552-313-02 12 in 1 CARTON 07/15/2011 2 2 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 02/12/2008 Labeler - Gil Pharmaceutical Corp (176826592)