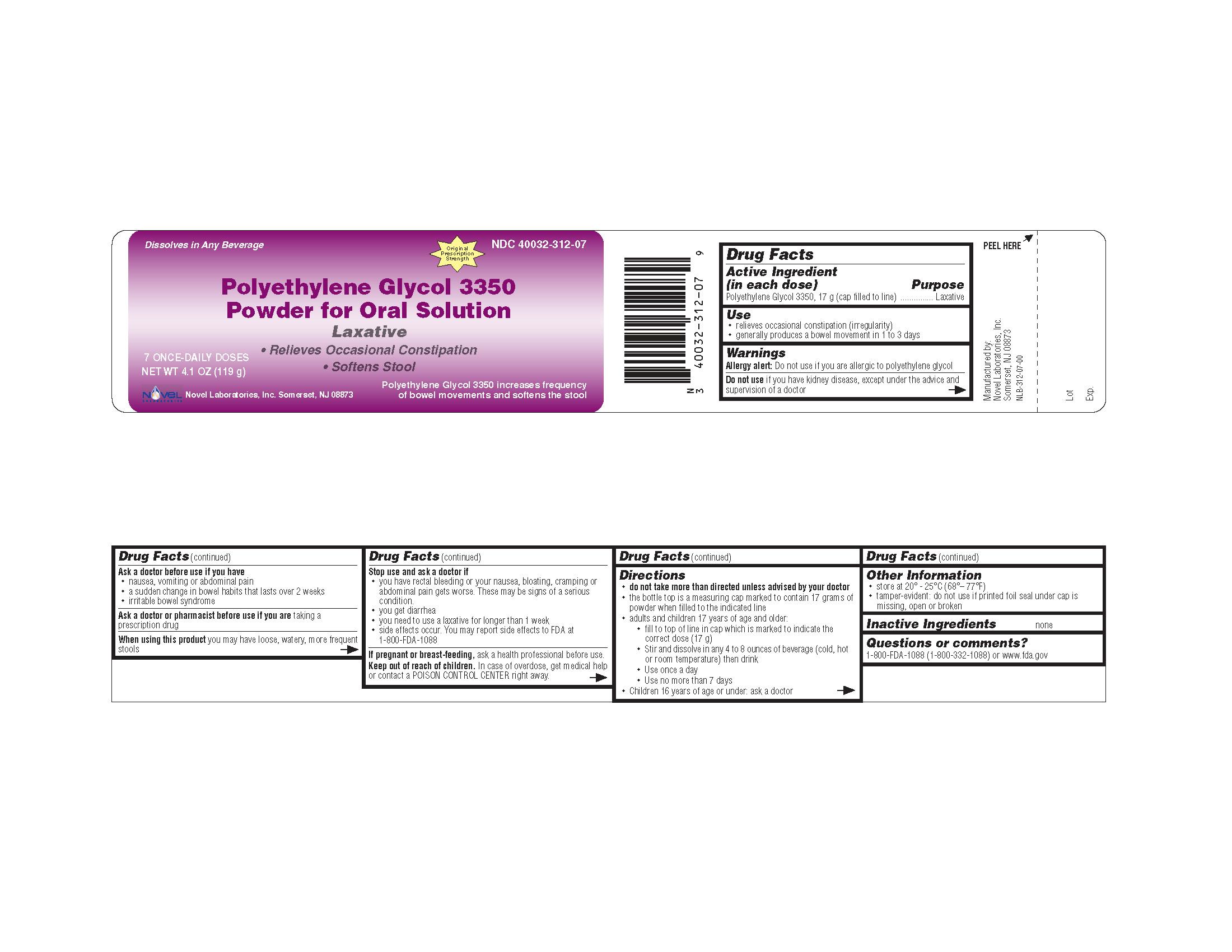
Label: POLYETHYLENE GLYCOL-3350 powder, for solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 40032-312-07, 40032-312-08, 40032-312-14 - Packager: Novel Laboratories, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated October 6, 2009
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- OTC - ACTIVE INGREDIENT
- WARNINGS
- OTC - DO NOT USE
- OTC - ASK DOCTOR
- OTC - ASK DOCTOR/PHARMACIST
- OTC - WHEN USING
-
OTC - STOP USE
Stop use and ask a doctor if
-
you have rectal bleeding or your nausea, bloating, cramping or abdominal pain gets worse. These may be signs of a serious condition.
-
you get diarrhea
-
you need to use a laxative for longer than 1 week
-
side effects occur. You may report side effects to FDA at 1-800-FDA-1088
Drug Facts (continued)
-
- OTC - PREGNANCY OR BREAST FEEDING
-
OTC - KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. In case of overdose, get medical help or contact a POISON CONTROL CENTER right away.
Directions
-
do not take more than directed unless advised by your doctor
-
the bottle top is a measuring cap marked to contain 17 grams of powder when filled to the indicated line.
-
adults and children 17 years of age and older:
-
fill to top of line in cap which is marked to indicate the correct dose (17 g)
-
stir and dissolve in any 4 to 8 ounces of beverage (cold, hot or room temperature) then drink
-
use once a day
-
use no more than 7 days
-
-
children 16 years of age or under: ask a doctor
Other Information
-
store at 20° - 25°C (68°– 77°F)
-
tamper-evident: do not use if printed foil seal under cap is missing, open or broken
-
- INACTIVE INGREDIENT
- OTC - QUESTIONS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
POLYETHYLENE GLYCOL-3350
polyethylene glycol-3350 powder, for solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:40032-312 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength POLYETHYLENE GLYCOL 3350 (UNII: G2M7P15E5P) (POLYETHYLENE GLYCOL 3350 - UNII:G2M7P15E5P) POLYETHYLENE GLYCOL 3350 17 g in 17 g Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:40032-312-07 119 g in 1 BOTTLE 2 NDC:40032-312-08 238 g in 1 BOTTLE 3 NDC:40032-312-14 510 g in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091077 10/06/2009 Labeler - Novel Laboratories, Inc. (793518643) Contact Address Telephone Number Email Address Anu Radha Subramanian Address: 400 Campus Drive City, State, Zip: Somerset, NJ, 08873 Country: USA ++1908-603-6000;ext=6002 ars@novellabs.net Registrant - Novel Laboratories, Inc. (793518643) Establishment Name Address ID/FEI Business Operations Novel Laboratories, Inc. 793518643 MANUFACTURE, ANALYSIS, repack Establishment Name Address ID/FEI Business Operations Dow Chemical Company 801038019 api manufacture