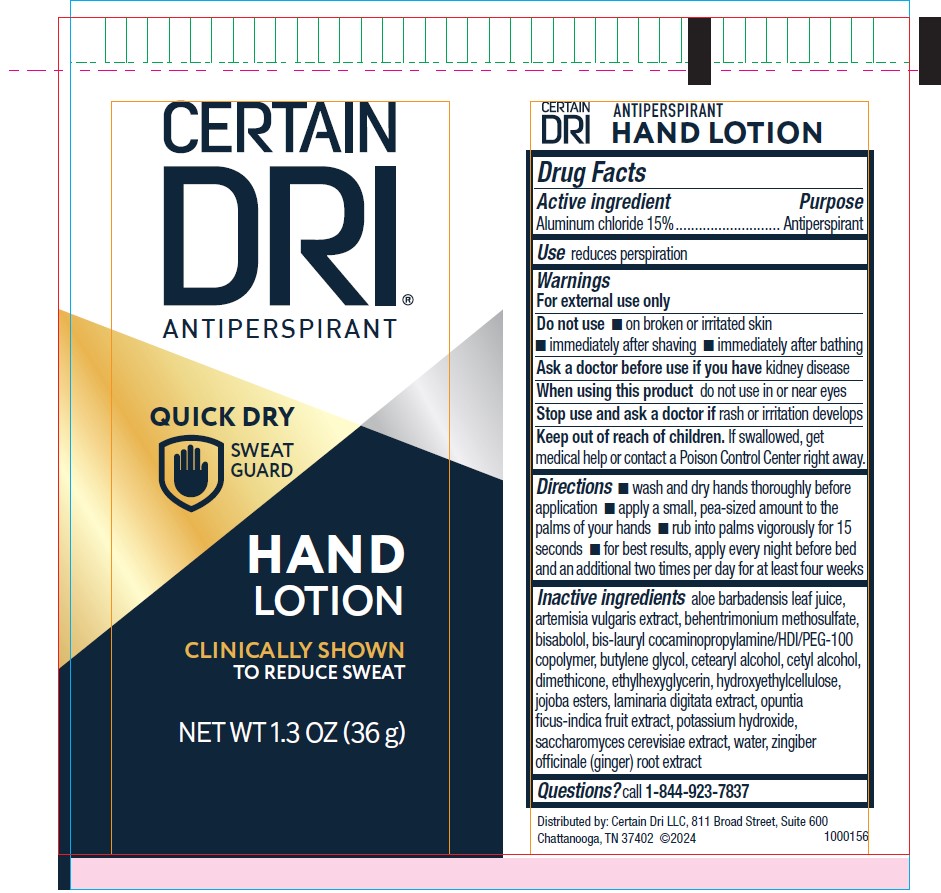
Label: CERTAIN DRI ANTIPERSPIRANT HAND-LOTION- aluminum chloride lotion
- NDC Code(s): 69693-723-13
- Packager: Clarion Brands, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated July 5, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
- Do not use
- ASK DOCTOR
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
Inactive ingredients
aloe barbadensis leaf juice, artemisia vulgarixs extract, behentrimonium methosulfate, bisaolol, bis-laurl cocaminopropylamine/HDI/PEG-100 copolymer, butylene glycol, cetearyl alcohol, cetyl alcohol, dimethicone, ethylhexyglycerin, hydroxyethylcellulose, jojoba esters, laminaria digitata extract, opuntia ficus-indica fruit extract, potassium hydroxide, saccharomyces cerevisiae extract, water, zingiber officinale (ginger) root extract
- Questions?
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
CERTAIN DRI ANTIPERSPIRANT HAND-LOTION
aluminum chloride lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69693-723 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Aluminum Chloride (UNII: 3CYT62D3GA) (Aluminum Cation - UNII:3XHB1D032B) Aluminum Chloride .15 g in 100 mL Inactive Ingredients Ingredient Name Strength Aloe Vera Leaf (UNII: ZY81Z83H0X) Artemisia Vulgaris Root (UNII: 32MP823R8S) Behentrimonium Methosulfate (UNII: 5SHP745C61) Levomenol (UNII: 24WE03BX2T) Thiamine Bis-Laurylsulfate (UNII: 226A8HU328) Butylene Glycol (UNII: 3XUS85K0RA) Cetostearyl Alcohol (UNII: 2DMT128M1S) Cetyl Alcohol (UNII: 936JST6JCN) Dimethicone (UNII: 92RU3N3Y1O) Ethylhexylglycerin (UNII: 147D247K3P) Hydroxyethyl Cellulose, Unspecified (UNII: T4V6TWG28D) Hydrolyzed Jojoba Esters (Acid Form) (UNII: UDR641JW8W) Laminaria Digitata (UNII: 15E7C67EE8) Opuntia Ficus-Indica Fruit Juice (UNII: 5ZC110ZY2H) Potassium Hydroxide (UNII: WZH3C48M4T) Saccharomyces Cerevisiae (UNII: 978D8U419H) Water (UNII: 059QF0KO0R) Ginger (UNII: C5529G5JPQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69693-723-13 1 in 1 CARTON 09/01/2024 1 36 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 09/01/2024 Labeler - Clarion Brands, LLC (079742703)