Label: METHADOSE- methadone hydrochloride concentrate
METHADOSE SUGAR-FREE- methadone hydrochloride concentrate
- NDC Code(s): 0406-0527-10, 0406-0527-15, 0406-8725-10, 0406-8725-15
- Packager: SpecGx LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: New Drug Application
Drug Label Information
Updated December 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use METHADOSE safely and effectively. See full prescribing information for METHADOSE.
METHADOSE (methadone hydrochloride) oral concentrate, CII
Initial U.S. Approval: 1947WARNING: LIFE-THREATENING RESPIRATORY DEPRESSION, LIFE-THREATENING QT PROLONGATION, ACCIDENTAL INGESTION, ABUSE POTENTIAL, INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES and TREATMENT FOR OPIOID ADDICTION
See full prescribing information for complete boxed warning.
- Fatal respiratory depression may occur, with highest risk at initiation and with dose increases. Instruct patients on proper administration of METHADOSE to reduce the risk. (5.1)
- Concomitant use with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, may result in profound sedation, respiratory depression, and death. (5.2, 7)
- QT Interval prolongation and serious arrhythmia (torsades de pointes) have occurred with treatment with methadone. (5.3)
- Accidental ingestion of METHADOSE can result in fatal overdose of methadone, especially in children. (5.4)
- METHADOSE contains methadone, a Schedule II controlled substance and can be abused and criminally diverted. (5.5)
- Concomitant use with CYP3A4, 2B6, 2C19, 2C9 or 2D6 inhibitors or discontinuation of concomitantly used CYP3A4, 2B6, 2C19, or 2C9 inducers can result in a fatal overdose of methadone. (5.7, 7)
-
Methadone products, when used for the treatment of opioid addiction in detoxification or maintenance programs, shall be dispensed only by certified opioid treatment programs as stipulated in 42 CFR 8.12. (2.1)
RECENT MAJOR CHANGES
Warnings and Precautions (5.17) 12/2023 INDICATIONS AND USAGE
METHADOSE is an opioid agonist indicated for the:
- Detoxification treatment of opioid addiction (heroin or other morphine-like drugs). (1)
- Maintenance treatment of opioid addiction (heroin or other morphine-like drugs), in conjunction with appropriate social and medical services. (1)
Limitations of Use
- Methadone products used for the treatment of opioid addiction in detoxification or maintenance programs are subject to the conditions for distribution and use required under 21 CFR, Title 42, Sec. 8. ( 2.1)
DOSAGE AND ADMINISTRATION
-
Strongly consider prescribing naloxone at the time METHADOSE is initiated or renewed because patients being treated with methadone may be at risk for opioid overdose during initiation or titration, or in the case of relapse to illicit use. (2.3)
-
Initiation of Detoxification and Maintenance Treatment: A single dose of 20 to 30 mg may be sufficient to suppress withdrawal syndrome. (2.4)
-
Maintenance Treatment: Clinical stability is most commonly achieved at doses between 80 to 120 mg/day. (2.5)
-
Do not abruptly discontinue METHADOSE in a physically dependent patient. (2.6, 5.15)
DOSAGE FORMS AND STRENGTHS
Oral concentrate: 10 mg/mL in 1-Liter bottle (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
-
Neonatal Opioid Withdrawal Syndrome: Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy. (5.6)
-
Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients: Monitor closely, particularly during initiation and titration. (5.8)
-
Serotonin Syndrome: Potentially life-threatening condition could result from concomitant serotonergic drug administration. Discontinue METHADOSE if serotonin syndrome is suspected. (5.9)
-
Adrenal Insufficiency: If diagnosed, treat with physiologic replacement of corticosteroids, and wean patient off of the opioid. (5.10)
-
Severe Hypotension: Monitor during dose initiation and titration. (5.11)
-
Risks of Use in Patients with Head Injury and Increased Intracranial Pressure: Monitor for sedation and respiratory depression. Avoid use of methadone in patients with impaired consciousness or coma susceptible to intracranial effects of CO2 retention. (5.12)
ADVERSE REACTIONS
Most Common Adverse Reactions Are: lightheadedness, dizziness, sedation, nausea, vomiting, and sweating. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Mallinckrodt at 1-800-778-7898 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
-
Potentially Arrhythmogenic Agents: Monitor patients closely for cardiac conduction changes. (7)
-
Interactions with CNS Depressants: Consider dose reduction of one or both drugs because of additive effects. (7)
-
Mixed Agonist/Antagonist and Partial Agonist Opioids: Avoid concomitant use with METHADOSE because it may precipitate withdrawal symptoms. (5.15, 7)
USE IN SPECIFIC POPULATIONS
-
Lactation: Monitor breastfed infants for increased drowsiness and breathing difficulties. (8.2)
Revised: 12/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: LIFE-THREATENING RESPIRATORY DEPRESSION, LIFE-THREATENING QT PROLONGATION, ACCIDENTAL INGESTION, ABUSE POTENTIAL, INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES and TREATMENT FOR OPIOID ADDICTION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Conditions for Distribution and Use of Methadone Products for the Treatment of Opioid Addiction
2.2 Important Dosage and Administration Information
2.3 Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
2.4 Induction/Initial Dosing for Detoxification and Maintenance Treatment of Opioid Addiction
2.5 Titration and Maintenance Treatment of Opioid Dependence
2.6 Medically Supervised Withdrawal After a Period of Maintenance Treatment for Opioid Addiction
2.7 Risk of Relapse in Patients on Methadone Maintenance Treatment of Opioid Addiction
2.8 Considerations for Management of Acute Pain During Methadone Maintenance Treatment
2.9 Dosage Adjustment During Pregnancy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Life-Threatening Respiratory Depression
5.2 Managing Risks from Concomitant Use of Benzodiazepines or Other CNS Depressants with Methadone
5.3 Life-Threatening QT Prolongation
5.4 Accidental Ingestion
5.5 Misuse, Abuse, and Diversion of Opioids
5.6 Neonatal Opioid Withdrawal Syndrome
5.7 Risks of Concomitant Use of Cytochrome P450 3A4, 2B6, 2C19, 2C9, or 2D6 Inhibitors or Discontinuation of P450 3A4, 2B6, 2C19, or 2C9 Inducers
5.8 Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients
5.9 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs
5.10 Adrenal Insufficiency
5.11 Severe Hypotension
5.12 Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness
5.13 Risks of Use in Patients with Gastrointestinal Conditions
5.14 Increased Risks of Seizure in Patients with Seizure Disorders
5.15 Withdrawal
5.16 Risks of Driving or Operating Machinery
5.17 Hypoglycemia
5.18 Laboratory Test Interactions
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
8.7 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: LIFE-THREATENING RESPIRATORY DEPRESSION, LIFE-THREATENING QT PROLONGATION, ACCIDENTAL INGESTION, ABUSE POTENTIAL, INTERACTIONS WITH DRUGS AFFECTING CYTOCHROME P450 ISOENZYMES and TREATMENT FOR OPIOID ADDICTION
Life-Threatening Respiratory Depression
Respiratory depression, including fatal cases, have been reported during initiation and conversion of patients to methadone, and even when the drug has been used as recommended and not misused or abused [see Warnings and Precautions (5.1)]. Proper dosing and titration are essential and METHADOSE should only be prescribed by healthcare professionals who are knowledgeable in the use of methadone for detoxification and maintenance treatment of opioid addiction. Monitor for respiratory depression, especially during initiation of METHADOSE or following a dose increase. The peak respiratory depressant effect of methadone occurs later, and persists longer than the peak pharmacologic effect, especially during the initial dosing period.Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants
Concomitant use with benzodiazepines or other central nervous system (CNS) depressants, including alcohol, is a risk factor for respiratory depression and death [see Warnings and Precautions (5.2)].- Reserve concomitant prescribing of benzodiazepines or other CNS depressants in patients in methadone treatment to those for whom alternatives to benzodiazepines or other CNS depressants are inadequate.
- Follow patients for signs and symptoms of respiratory depression and sedation. If the patient is visibly sedated, evaluate the cause of sedation and consider delaying or omitting daily methadone dosing.
Life-Threatening QT Prolongation
QT interval prolongation and serious arrhythmia (torsades de pointes) have occurred during treatment with methadone [see Warnings and Precautions (5.3)]. Most cases involve patients being treated for pain with large, multiple daily doses of methadone, although cases have been reported in patients receiving doses commonly used for maintenance treatment of opioid addiction. Closely monitor patients with risk factors for development of prolonged QT interval, a history of cardiac conduction abnormalities, and those taking medications affecting cardiac conduction for changes in cardiac rhythm during initiation and titration of METHADOSE.Accidental Ingestion
Accidental ingestion of METHADOSE, especially by children, can result in fatal overdose of methadone [see Warnings and Precautions (5.4)].Misuse, Abuse, and Diversion of Opioids
METHADOSE contains methadone, an opioid agonist and Schedule II controlled substance with an abuse liability similar to other opioid agonists, legal or illicit [see Warnings and Precautions (5.5)].Interactions with Drugs Affecting Cytochrome P450 Isoenzymes
The concomitant use of METHADOSE with all cytochrome P450 3A4, 2B6, 2C19, 2C9 or 2D6 inhibitors may result in an increase in methadone plasma concentrations, which could cause potentially fatal respiratory depression. In addition, discontinuation of concomitantly used cytochrome P450 3A4 2B6, 2C19, or 2C9 inducers may also result in an increase in methadone plasma concentration. Follow patients closely for respiratory depression and sedation, and consider dosage reduction with any changes of concomitant medications that can result in an increase in methadone levels [see Warnings and Precautions (5.7), Drug Interactions (7)].
Conditions for Distribution and Use of Methadone Products for the Treatment of Opioid Addiction
For detoxification and maintenance of opioid dependence, methadone should be administered in accordance with the treatment standards cited in 42 CFR Section 8, including limitations on unsupervised administration [see Dosage and Administration (2.1)]. -
1 INDICATIONS AND USAGE
METHADOSE contains methadone, an opioid agonist indicated for the:
- detoxification treatment of opioid addiction (heroin or other morphine-like drugs).
- maintenance treatment of opioid addiction (heroin or other morphine-like drugs), in conjunction with appropriate social and medical services.
Limitations of Use
Methadone products used for the treatment of opioid addiction in detoxification or maintenance programs are subject to the conditions for distribution and use required under 21 CFR, Title 42, Sec 8 [see Dosage and Administration (2.1)].
-
2 DOSAGE AND ADMINISTRATION
2.1 Conditions for Distribution and Use of Methadone Products for the Treatment of Opioid Addiction
Code of Federal Regulations, Title 42, Sec 8: Methadone products when used for the treatment of opioid addiction in detoxification or maintenance programs, shall be dispensed only by opioid treatment programs (and agencies, practitioners or institutions by formal agreement with the program sponsor) certified by the Substance Abuse and Mental Health Services Administration and approved by the designated state authority. Certified treatment programs shall dispense and use methadone in oral form only and according to the treatment requirements stipulated in the Federal Opioid Treatment Standards (42 CFR 8.12). See below for important regulatory exceptions to the general requirement for certification to provide opioid agonist treatment.
Failure to abide by the requirements in these regulations may result in criminal prosecution, seizure of the drug supply, revocation of the program approval, and injunction precluding operation of the program.
Regulatory Exceptions to the General Requirement for Certification to Provide Opioid Agonist Treatment
- During inpatient care, when the patient was admitted for any condition other than concurrent opioid addiction (pursuant to 21 CFR 1306.07(c)), to facilitate the treatment of the primary admitting diagnosis.
- During an emergency period of no longer than 3 days while definitive care for the addiction is being sought in an appropriately licensed facility (pursuant to 21 CFR 1306.07(b)).
2.2 Important Dosage and Administration Information
METHADOSE is for oral administration only. The preparation must not be injected. Package in child-resistant containers and inform patients that METHADOSE should be kept out of reach of children to prevent accidental ingestion [see Patient Counseling Information (17)].
Consider the following important factors that differentiate methadone from other opioids:
- The peak respiratory depressant effect of methadone occurs later and persists longer than its peak pharmacologic effect.
- A high degree of opioid tolerance does not eliminate the possibility of methadone overdose, iatrogenic or otherwise. Deaths have been reported during conversion to methadone from chronic, high-dose treatment with other opioid agonists and during initiation of methadone treatment of addiction in subjects previously abusing high doses of other opioid agonists.
- There is high interpatient variability in absorption, metabolism, and relative analgesic potency. Population-based conversion ratios between methadone and other opioids are not accurate when applied to individuals.
- With repeated dosing, methadone is retained in the liver and then slowly released, prolonging the duration of potential toxicity.
- Steady-state plasma concentrations are not attained until 3 to 5 days after initiation of dosing.
METHADOSE has a narrow therapeutic index, especially when combined with other drugs.
2.3 Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver. Because patients being treated with methadone may be at risk for opioid overdose during initiation or titration, or in the case of relapse to illicit use, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, both when initiating and renewing treatment with METHADOSE. Also consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose [see Warnings and Precautions (5.1)].
Advise patients and caregivers that naloxone may also be administered for a known or suspected overdose with METHADOSE itself [see Overdosage (10)].
Inform patients and caregivers of their options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing regulations (e.g., by prescription, directly from a pharmacist, or as part of a community-based program) [see Patient Counseling Information (17)].
2.4 Induction/Initial Dosing for Detoxification and Maintenance Treatment of Opioid Addiction
For detoxification and maintenance of opiate dependence, methadone should be administered in accordance with the treatment standards cited in 42 CFR Section 8.12, including limitations on unsupervised administration. Administer the initial methadone dose under supervision, when there are no signs of sedation or intoxication, and the patient shows symptoms of withdrawal. An initial single dose of 20 to 30 mg of methadone will often be sufficient to suppress withdrawal symptoms. The initial dose should not exceed 30 mg.
To make same-day dosing adjustments, have the patient wait 2 to 4 hours for further evaluation, when peak levels have been reached. Provide an additional 5 to 10 mg of methadone if withdrawal symptoms have not been suppressed or if symptoms reappear.
The total daily dose of methadone on the first day of treatment should not ordinarily exceed 40 mg. Adjust the dose over the first week of treatment based on control of withdrawal symptoms at the time of expected peak activity (i.e., 2 to 4 hours after dosing). When adjusting the dose, keep in mind that methadone will accumulate over the first several days of dosing; deaths have occurred in early treatment due to the cumulative effects. Instruct patients that the dose will “hold” for a longer period of time as tissue stores of methadone accumulate.
Use lower initial doses for patients whose tolerance is expected to be low at treatment entry. Any patient who has not taken opioids for more than 5 days may no longer be tolerant. Do not determine initial doses based on previous treatment episodes or dollars spent per day on illicit drug use. Also consider concurrent medications and the general condition and medical status of the patient when selecting the initial dose.
During the induction phase of methadone maintenance treatment, patients are being withdrawn from other opioids and may show typical withdrawal symptoms. Monitor patients for signs and symptoms of opioid withdrawal including: lacrimation, rhinorrhea, sneezing, yawning, excessive perspiration, goose-flesh, fever, chilliness alternating with flushing, restlessness, irritability, weakness, anxiety, depression, dilated pupils, tremors, tachycardia, abdominal cramps, body aches, involuntary twitching and kicking movements, anorexia, nausea, vomiting, diarrhea, intestinal spasms, and weight loss and consider dose adjustment as indicated.
Short-Term Detoxification
For a brief course of stabilization followed by a period of medically supervised withdrawal, titrate the patient to a total daily dose of about 40 mg in divided doses to achieve an adequate stabilizing level. After 2 to 3 days of stabilization, gradually decrease the dose of methadone. Decrease the dose of methadone on a daily basis or at 2-day intervals, keeping the amount of methadone sufficient to keep withdrawal symptoms at a tolerable level. Hospitalized patients may tolerate a daily reduction of 20% of the total daily dose. Ambulatory patients may need a slower schedule.
2.5 Titration and Maintenance Treatment of Opioid Dependence
Titrate patients in maintenance treatment to a dose that prevents opioid withdrawal symptoms for 24 hours, reduces drug hunger or craving, and blocks or attenuates the euphoric effects of self-administered opioids, ensuring that the patient is tolerant to the sedative effects of methadone. Most commonly, clinical stability is achieved at doses between 80 to 120 mg/day. During prolonged administration of methadone, monitor patients for persistent constipation and manage accordingly.
2.6 Medically Supervised Withdrawal After a Period of Maintenance Treatment for Opioid Addiction
There is considerable variability in the appropriate rate of methadone taper in patients choosing medically supervised withdrawal from methadone treatment. Dose reductions should generally be less than 10% of the established tolerance or maintenance dose, and 10- to 14-day intervals should elapse between dose reductions. Apprise patients of the high risk of relapse to illicit drug use associated with discontinuation of methadone maintenance treatment. Do not abruptly discontinue METHADOSE in a physically dependent patient [see Warnings and Precautions (5.15)].
2.7 Risk of Relapse in Patients on Methadone Maintenance Treatment of Opioid Addiction
Abrupt opioid discontinuation can lead to development of opioid withdrawal symptoms [see Drug Abuse and Dependence (9.3)]. Opioid withdrawal symptoms have been associated with an increased risk of relapse to illicit drug use in susceptible patients.
2.8 Considerations for Management of Acute Pain During Methadone Maintenance Treatment
Patients in methadone maintenance treatment for opioid dependence who experience physical trauma, postoperative pain, or other acute pain cannot be expected to derive analgesia from their existing dose of methadone. Such patients should be administered analgesics, including opioids, in doses that would otherwise be indicated for non-methadone-treated patients with similar painful conditions. When opioids are required for management of acute pain in methadone maintenance patients, somewhat higher and/or more frequent doses will often be required than would be the case for non-tolerant patients due to the opioid tolerance induced by methadone.
2.9 Dosage Adjustment During Pregnancy
Methadone clearance may be increased during pregnancy. During pregnancy, a woman’s methadone dose may need to be increased or the dosing interval decreased [see Use in Specific Populations (8.1)].
-
3 DOSAGE FORMS AND STRENGTHS
Oral Concentrate:
- 10 mg methadone hydrochloride (equivalent to 8.95 mg of methadone) per mL (10 mg/mL) as a red, cherry-flavored liquid concentrate in 1-liter bottles
- 10 mg methadone hydrochloride (equivalent to 8.95 mg of methadone) per mL (10 mg/mL) as a dye-free, sugar-free, unflavored, clear liquid concentrate in 1-liter bottles
-
4 CONTRAINDICATIONS
METHADOSE is contraindicated in patients with:
-
Significant respiratory depression [see Warnings and Precautions (5.1)]
-
Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment [see Warnings and Precautions (5.8)]
-
Known or suspected gastrointestinal obstruction, including paralytic ileus [see Warnings and Precautions (5.13)]
-
Hypersensitivity (e.g., anaphylaxis) to methadone or any other ingredient in METHADOSE [see Adverse Reactions (6)]
-
-
5 WARNINGS AND PRECAUTIONS
5.1 Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression has been reported with the use of methadone, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Respiratory depression from opioids is manifested by a reduced urge to breathe and a decreased rate of respiration, often associated with a “sighing” pattern of breathing (deep breaths separated by abnormally long pauses). Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status [see Overdosage (10)].
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of METHADOSE, the risk is greatest during the initiation of therapy or following a dose increase. The peak respiratory depressant effect of methadone occurs later, and persists longer than the peak pharmacologic effect, especially during the initial dosing period. Monitor patients closely for respiratory depression, when initiating therapy with METHADOSE and following dose increases.
Instruct patients against use by individuals other than the patient for whom methadone was prescribed and to keep methadone out of the reach of children, as such inappropriate use may result in fatal respiratory depression [see Patient Counseling Information (17)].
To reduce the risk of respiratory depression, proper dosing and titration of methadone are essential [see Dosage and Administration (2.4)]. Overestimating the methadone dosage when initiating treatment can result in fatal overdose with the first dose.
To further reduce the risk of respiratory depression, consider the following:
- Patients tolerant to other opioids may be incompletely tolerant to methadone. Incomplete cross-tolerance is of particular concern for patients tolerant to other mu-opioid agonists. Deaths have been reported during conversion from chronic, high-dose treatment with other opioid agonists. Follow induction directions closely to avoid inadvertent overdose [see Dosage and Administration (2.4)].
- Proper dosing and titration are essential and methadone should be overseen only by healthcare professionals who are knowledgeable in the pharmacokinetics and pharmacodynamics of methadone.
Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help right away in the event of a known or suspected overdose [see Patient Counseling Information (17)].
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see Dosage and Administration (2)].
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Discuss the availability of naloxone for the emergency treatment of opioid overdose with the patient and caregiver. Because patients being treated with methadone may be at risk for opioid overdose during initiation or titration, or in the case of relapse to illicit use, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, both when initiating and renewing treatment with METHADOSE. Also consider prescribing naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose [see Dosage and Administration (2.3)].Advise patients and caregivers that naloxone may also be administered for a known or suspected overdose with METHADOSE itself [see Overdosage (10)].
Inform patients and caregivers of their options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements or guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program). Educate patients and caregivers on how to recognize respiratory depression and, if naloxone is prescribed, how to treat with naloxone. Emphasize the importance of calling 911 or getting emergency medical help, even if naloxone is administered [see Patient Counseling Information (17)].
5.2 Managing Risks from Concomitant Use of Benzodiazepines or Other CNS Depressants with Methadone
Concomitant use of methadone and benzodiazepines or other CNS depressants increases the risk of adverse reactions including overdose and death. Medication-assisted treatment of opioid use disorder, however, should not be categorically denied to patients taking these drugs. Prohibiting or creating barriers to treatment can pose an even greater risk of morbidity and mortality due to the opioid use disorder alone.
As a routine part of orientation to methadone treatment, educate patients about the risks of concomitant use of benzodiazepines, sedatives, opioid analgesics, or alcohol.
Develop strategies to manage use of prescribed or illicit benzodiazepines or other CNS depressants at admission to methadone treatment, or if it emerges as a concern during treatment. Adjustments to induction procedures and additional monitoring may be required. There is no evidence to support dose limitations or arbitrary caps of methadone as a strategy to address benzodiazepine use in methadone-treated patients. However, if a patient is sedated at the time of methadone dosing, ensure that a medically-trained healthcare provider evaluates the cause of sedation and delays or omits the methadone dose if appropriate.
Cessation of benzodiazepines or other CNS depressants is preferred in most cases of concomitant use. In some cases monitoring in a higher level of care for taper may be appropriate. In others, gradually tapering a patient off a prescribed benzodiazepine or other CNS depressant or decreasing to the lowest effective dose may be appropriate.
For patients in methadone treatment, benzodiazepines are not the treatment of choice for anxiety or insomnia. Before co-prescribing benzodiazepines, ensure that patients are appropriately diagnosed and consider alternative medications and non-pharmacologic treatments to address anxiety or insomnia. Ensure that other healthcare providers prescribing benzodiazepines or other CNS depressants are aware of the patient’s methadone treatment and coordinate care to minimize the risks associated with concomitant use.
If concomitant use is warranted, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, as is recommended for all patients in methadone treatment for opioid use disorder [see Warnings and Precautions (5.1)].
In addition, take measures to confirm that patients are taking the medications prescribed and not diverting or supplementing with illicit drugs. Toxicology screening should test for prescribed and illicit benzodiazepines [see Drug Interactions (7)].
5.3 Life-Threatening QT Prolongation
Cases of QT interval prolongation and serious arrhythmia (torsades de pointes) have been observed during treatment with methadone. These cases appear to be more commonly associated with, but not limited to, higher dose treatment (> 200 mg/day). Most cases involve patients being treated for pain with large, multiple daily doses of methadone, although cases have been reported in patients receiving doses commonly used for maintenance treatment of opioid addiction. In most patients on the lower doses typically used for maintenance, concomitant medications and/or clinical conditions such as hypokalemia were noted as contributing factors. However, the evidence strongly suggests that methadone possesses the potential for adverse cardiac conduction effects in some patients. The effects of methadone on the QT interval have been confirmed in in vivo laboratory studies, and methadone has been shown to inhibit cardiac potassium channels in in vitro studies.
Closely monitor patients with risk factors for development of prolonged QT interval (e.g., cardiac hypertrophy, concomitant diuretic use, hypokalemia, hypomagnesemia), a history of cardiac conduction abnormalities, and those taking medications affecting cardiac conduction. QT prolongation has also been reported in patients with no prior cardiac history who have received high doses of methadone.
Evaluate patients developing QT prolongation while on METHADOSE treatment for the presence of modifiable risk factors, such as concomitant medications with cardiac effects, drugs which might cause electrolyte abnormalities, and drugs which might act as inhibitors of methadone metabolism.
Only initiate therapy with METHADOSE in patients for whom the anticipated benefit outweighs the risk of QT prolongation and development of dysrhythmias that have been reported with high doses of methadone. The use of methadone in patients already known to have a prolonged QT interval has not been systematically studied.
5.4 Accidental Ingestion
Accidental ingestion of even one dose of METHADOSE, especially by children, can result in respiratory depression and death due to an overdose. Keep METHADOSE out of reach of children to prevent accidental ingestion [see Warnings and Precautions (5.1)].
5.5 Misuse, Abuse, and Diversion of Opioids
METHADOSE contains methadone, an opioid agonist and a Schedule II controlled substance. Methadone can be abused in a manner similar to other opioid agonists, legal or illicit. Opioid agonists are sought by and people with opioid use disorders and are subject to criminal diversion.
Contact local state professional licensing board or state-controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
5.6 Neonatal Opioid Withdrawal Syndrome
Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy, whether that use is medically-authorized or illicit. Unlike opioid withdrawal syndrome in adults, NOWS may be life-threatening if not recognized and treated in the neonate. Healthcare professionals should observe newborns for signs of NOWS and manage accordingly [see Use in Specific Populations (8.1)].
Advise pregnant women receiving opioid addiction treatment with METHADOSE of the risk of neonatal opioid withdrawal syndrome and ensure that appropriate treatment will be available [see Use in Specific Populations (8.1)]. This risk must be balanced against the risk of untreated opioid addiction which often results in continued or relapsing illicit opioid use and is associated with poor pregnancy outcomes. Therefore, prescribers should discuss the importance and benefits of management of opioid addiction throughout pregnancy.
5.7 Risks of Concomitant Use of Cytochrome P450 3A4, 2B6, 2C19, 2C9, or 2D6 Inhibitors or Discontinuation of P450 3A4, 2B6, 2C19, or 2C9 Inducers
Concomitant use of METHADOSE with CYP3A4, CYP2B6, CYP2C19, CYP2C9, or CYP2D6 inhibitors, may increase plasma concentrations of methadone, prolong opioid adverse reactions, and may cause potentially fatal respiratory depression, particularly when an inhibitor is added after a stable dose of METHADOSE is achieved. Similarly, discontinuation of concomitant CYP3A4, CYP2B6, CYP2C19, or CYP2C9 inducers in METHADOSE-treated patients may increase methadone plasma concentrations resulting in fatal respiratory depression. Consider dosage reduction of METHADOSE when using concomitant CYP3A4, CYP2B6, CYP2C19, CYP2C9 or CYP2D6 inhibitors or discontinuing CYP3A4, CYP2B6, CYP2C19, or CYP2C9 inducers in methadone-treated patients, and follow patients closely at frequent intervals for signs and symptoms of respiratory depression and sedation [see Drug Interactions (7)].
Addition of CYP3A4, CYP2B6, CYP2C19, or CYP2C9 inducers or discontinuation of a CYP3A4, CYP2B6, CYP2C19, CYP2C9, or CYP2D6 inhibitors in patients treated with METHADOSE may decrease methadone plasma concentrations, reducing efficacy and may lead to opioid withdrawal symptoms in patients physically dependent on methadone. When using METHADOSE with CYP3A4, CYP2B6, CYP2C19, or CYP2C9 inducers or discontinuing CYP3A4, CYP2B6, CYP2C19, CYP2C9, or CYP2D6 inhibitors, follow patients for signs or symptoms of opioid withdrawal and consider increasing the METHADOSE dosage as needed [see Drug Interactions (7)].
5.8 Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated Patients
The use of METHADOSE in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease
METHADOSE-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with a substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive including apnea, even at recommended dosages of METHADOSE [see Warnings and Precautions (5.1)].Elderly, Cachectic, or Debilitated Patients
Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients because they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients [see Warnings and Precautions (5.1)].Monitor such patients closely, particularly when initiating and titrating METHADOSE and when METHADOSE is given concomitantly with other drugs that depress respiration [see Warnings and Precautions (5.2)].
5.9 Serotonin Syndrome with Concomitant Use of Serotonergic Drugs
Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of METHADOSE with serotonergic drugs. Serotonergic drugs include selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonergic neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), and drugs that impair metabolism of serotonin (including MAO inhibitors, both those intended to treat psychiatric disorders and others, such as linezolid and intravenous methylene blue) [see Drug Interactions (7)]. This may occur within the recommended dosage range.
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination, rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms generally occurs within several hours to a few days of concomitant use, but may occur later than that. Discontinue METHADOSE if serotonin syndrome is suspected.
5.10 Adrenal Insufficiency
Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
5.11 Severe Hypotension
Methadone may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is an increased risk in patients whose ability to maintain normal blood pressure is compromised by a reduced blood volume or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics) [see Drug Interactions (7)]. Monitor these patients for signs of hypotension after initiating or titrating the dosage of METHADOSE. In patients with circulatory shock, METHADOSE may cause vasodilation that can further reduce cardiac output and blood pressure. Avoid the use of METHADOSE in patients with circulatory shock.
5.12 Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired Consciousness
In patients who may be susceptible to the intracranial effects of CO2 retention (e.g., those with evidence of increased intracranial pressure or brain tumors), METHADOSE may reduce respiratory drive, and the resultant CO2 retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with methadone. Opioids may also obscure the clinical course in a patient with a head injury.
Avoid the use of methadone in patients with impaired consciousness or coma.
5.13 Risks of Use in Patients with Gastrointestinal Conditions
METHADOSE is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus. The methadone in METHADOSE may cause spasm of the sphincter of Oddi. Opioids may cause increases in the serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
5.14 Increased Risks of Seizure in Patients with Seizure Disorders
Methadone may increase frequency of seizures in patients with seizure disorders and increase the risks of seizures occurring in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during METHADOSE therapy.
5.15 Withdrawal
Avoid the use of mixed agonist/antagonist (i.e., pentazocine, nalbuphine, and butorphanol) or partial agonist (e.g., buprenorphine) analgesics in patients who are receiving a full opioid agonist, including METHADOSE. In these patients, mixed agonists/antagonist and partial agonist analgesics may precipitate withdrawal symptoms [see Drug Interactions (7)].
When discontinuing METHADOSE, gradually taper the dosage [see Dosage and Administration (2.6, 2.7)]. Do not abruptly discontinue METHADOSE [see Drug Abuse and Dependence (9.3)].
5.16 Risks of Driving or Operating Machinery
METHADOSE may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of METHADOSE and know how they will react to the medication [see Patient Counseling Information (17)].
5.17 Hypoglycemia
Cases of methadone-associated hypoglycemia have been reported, some resulting in hospitalization. In many cases, patients had predisposing risk factors (e.g., diabetes). The relationship between methadone and hypoglycemia is not fully understood but may be dose dependent. If hypoglycemia is suspected, monitor blood glucose levels, and manage the patient as clinically appropriate.
-
6 ADVERSE REACTIONS
The following serious adverse reactions and/or conditions are described, or described in greater detail, in other sections:
- Respiratory Depression [see Warnings and Precautions (5.1)]
- Interactions with Benzodiazepines and other CNS Depressants [see Warnings and Precautions (5.2)]
- QT Prolongation [see Warnings and Precautions (5.3)]
- Serotonin Syndrome [see Warnings and Precautions (5.9)]
- Adrenal Insufficiency [see Warnings and Precautions (5.10)]
- Severe Hypotension [see Warnings and Precautions (5.11)]
- Gastrointestinal Adverse Reactions [see Warnings and Precautions (5.13)]
- Seizures [see Warnings and Precautions (5.14)]
- Withdrawal [see Warnings and Precautions (5.15)]
- Hypoglycemia [see Warnings and Precautions (5.17)]
The following adverse reactions have been identified during post-approval use of methadone. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The major hazards of methadone are respiratory depression and, to a lesser degree, systemic hypotension. Respiratory arrest, shock, cardiac arrest, and death have occurred.
The most frequently observed adverse reactions include lightheadedness, dizziness, sedation, nausea, vomiting, and sweating. These effects seem to be more prominent in ambulatory patients and in those who are not suffering severe pain.
Other adverse reactions include the following:
Body as a Whole: asthenia (weakness), edema, headache
Cardiovascular: arrhythmias, bigeminal rhythms, bradycardia, cardiomyopathy, ECG abnormalities, extrasystoles, flushing, heart failure, hypotension, palpitations, phlebitis, QT interval prolongation, syncope, T-wave inversion, tachycardia, torsade de pointes, ventricular fibrillation, ventricular tachycardia
Central Nervous System: agitation, confusion, disorientation, dysphoria, euphoria, insomnia, hallucinations, seizures, visual disturbances, congenital oculomotor disorders (nystagmus, strabismus)
Endocrine: hypogonadism
Gastrointestinal: abdominal pain, anorexia, biliary tract spasm, constipation, dry mouth, glossitis
Hematologic: Reversible thrombocytopenia has been described in opioid addicts with chronic hepatitis.
Metabolic: hypokalemia, hypomagnesemia, weight gain
Musculoskeletal: decreased muscle mass and strength, osteoporosis and fractures
Renal: antidiuretic effect, urinary retention or hesitancy
Reproductive: amenorrhea, reduced libido and/or potency, reduced ejaculate volume, reduced seminal vesicle and prostate secretions, decreased sperm motility, abnormalities in sperm morphology
Respiratory: pulmonary edema, respiratory depression
Skin and Subcutaneous Tissue: pruritus, urticaria, other skin rashes, and rarely, hemorrhagic urticaria
Hypersensitivity: Anaphylaxis has been reported with ingredients contained in METHADOSE.
Serotonin syndrome: Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
Adrenal insufficiency: Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
Androgen deficiency: Cases of androgen deficiency have occurred with chronic use of opioids [see Clinical Pharmacology (12.2)].
Hypoglycemia: Cases of hypoglycemia have been reported in patients taking methadone [see Warnings and Precautions (5.17)].
-
7 DRUG INTERACTIONS
Benzodiazepines and Other Central Nervous System (CNS) Depressants
Clinical Impact:
Due to additive pharmacologic effect, the concomitant use of benzodiazepines or other CNS depressants, including alcohol, increases the risk of respiratory depression, profound sedation, coma, and death.
Intervention:
Cessation of benzodiazepines or other CNS depressants is preferred in most cases of concomitant use. In some cases, monitoring in a higher level of care for taper may be appropriate. In others, gradually tapering a patient off of a prescribed benzodiazepine or other CNS depressant or decreasing to the lowest effective dose may be appropriate.
Before co-prescribing benzodiazepines for anxiety or insomnia, ensure that patients are appropriately diagnosed and consider alternative medications and non-pharmacologic treatments [see Warnings and Precautions (5.2)].
If concomitant use is warranted, strongly consider prescribing naloxone for the emergency treatment of opioid overdose, as is recommended for all patients in treatment for opioid use disorder [see Warnings and Precautions (5.1)].
Examples:
Alcohol, benzodiazepines, and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids.
Inhibitors of CYP3A4, CYP2B6, CYP2C19, CYP2C9, or CYP2D6
Clinical Impact:
Methadone undergoes hepatic N-demethylation by several cytochrome P450 (CYP) isoforms, including CYP3A4, CYP2B6, CYP2C19, CYP2C9, and CYP2D6. The concomitant use of methadone and CYP3A4, CYP2B6, CYP2C19, CYP2C9, or CYP2D6 inhibitors can increase the plasma concentration of methadone, resulting in increased or prolonged opioid effects, and may result in a fatal overdose, particularly when an inhibitor is added after a stable dose of methadone is achieved. These effects may be more pronounced with concomitant use of drugs that inhibit more than one of the CYP enzymes listed above.
After stopping a CYP3A4, CYP2B6, CYP2C19, CYP2C9, or CYP2D6 inhibitor, as the effects of the inhibitor decline, the methadone plasma concentration can decrease [see Clinical Pharmacology (12.3)], resulting in decreased opioid efficacy or withdrawal symptoms in patients physically dependent on methadone.
Intervention:
If concomitant use is necessary, consider dosage reduction of methadone until stable drug effects are achieved. Monitor patients for respiratory depression and sedation at frequent intervals.
If a CYP3A4, CYP2B6, CYP2C19, CYP2C9, or CYP2D6 inhibitor is discontinued, follow patients for signs of opioid withdrawal and consider increasing the methadone dosage until stable drug effects are achieved.
Examples:
Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), protease inhibitors (e.g., ritonavir), fluconazole, fluvoxamine, some selective serotonin reuptake inhibitors (SSRIs) (e.g., sertraline, fluvoxamine)
Inducers of CYP3A4, CYP2B6, CYP2C19, or CYP2C9
Clinical Impact:
The concomitant use of methadone and CYP3A4, CYP2B6, CYP2C19, or CYP2C9 inducers can decrease the plasma concentration of methadone [see Clinical Pharmacology (12.3)], resulting in decreased efficacy or onset of withdrawal symptoms in patients physically dependent on methadone. These effects could be more pronounced with concomitant use of drugs that can induce multiple CYP enzymes.
After stopping a CYP3A4, CYP2B6, CYP2C19, or CYP2C9 inducer, as the effects of the inducer decline, the methadone plasma concentration can increase [see Clinical Pharmacology (12.3)], which could increase or prolong both the therapeutic effects and adverse reactions, and may cause serious respiratory depression, sedation, or death.
Intervention:
If concomitant use is necessary, consider increasing the methadone dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal. If a CYP3A4, CYP2B6, CYP2C19, or CYP2C9 inducer is discontinued, consider methadone dosage reduction and monitor for signs of respiratory depression and sedation.
Examples:
Rifampin, carbamazepine, phenytoin, St. John’s Wort, Phenobarbital
Potentially Arrhythmogenic Agents
Clinical Impact:
Pharmacodynamic interactions may occur with concomitant use of methadone and potentially arrhythmogenic agents or drugs capable of inducing electrolyte disturbances (hypomagnesemia, hypokalemia).
Intervention:
Monitor patients closely for cardiac conduction changes.
Examples:
Drugs known to have potential to prolong QT interval: Class I and III antiarrhythmics, some neuroleptics and tricyclic antidepressants, and calcium channel blockers. Drugs capable of inducing electrolyte disturbances: Diuretics, laxatives, and, in rare cases, mineralocortocoid hormones.
Serotonergic Drugs
Clinical Impact:
The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome [see Warnings and Precautions (5.9)].
Intervention:
If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue METHADOSE if serotonin syndrome is suspected.
Examples:
Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
Monoamine Oxidase Inhibitors (MAOIs)
Clinical Impact:
MAOI interactions with opioids may manifest as serotonin syndrome or opioid toxicity (e.g., respiratory depression, coma) [see Warnings and Precautions (5.1, 5.9)].
Intervention:
The use of METHADOSE is not recommended for patients taking MAOIs or within 14 days of stopping such treatment.
Examples:
phenelzine, tranylcypromine, linezolid
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics
Clinical Impact:
Patients maintained on methadone may experience withdrawal symptoms when given opioid antagonists, mixed agonist/antagonists, and partial agonists.
Intervention:
Avoid concomitant use.
Examples:
butorphanol, nalbuphine, pentazocine, buprenorphine
Muscle Relaxants
Clinical Impact:
Methadone may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression.
Intervention:
Monitor patients for signs of respiratory depression that may be greater than otherwise expected and decrease the dosage of METHADOSE and/or the muscle relaxant as necessary. Due to the risk of respiratory depression with concomitant use of skeletal muscle relaxants and opioids, strongly consider prescribing naloxone for the emergency treatment of opioid overdose [see Dosage and Administration (2.3), Warnings and Precautions (5.1, 5.2)].
Diuretics
Clinical Impact:
Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone.
Intervention:
Monitor patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed.
Anticholinergic Drugs
Clinical Impact:
The concomitant use of anticholinergic drugs may increase risk of urinary retention and/or severe constipation, which may lead to paralytic ileus.
Intervention:
Monitor patients for signs of urinary retention or reduced gastric motility when METHADOSE is used concomitantly with anticholinergic drugs.
Paradoxical Effects of Anti-Retroviral Agents on Methadone
Concurrent use of certain protease inhibitors with CYP3A4 inhibitory activity, alone and in combination, such as abacavir, amprenavir, darunavir+ritonavir, efavirenz, nelfinavir, nevirapine, ritonavir, telaprevir, lopinavir+ritonavir, saquinavir+ritonavir, and tipranvir+ritonavir, has resulted in increased clearance or decreased plasma levels of methadone. This may result in reduced efficacy of METHADOSE and could precipitate a withdrawal syndrome. Monitor patients receiving METHADOSE and any of these anti-retroviral therapies closely for evidence of withdrawal effects and adjust the METHADOSE dose accordingly.Effects of Methadone on Anti-Retroviral Agents
Didanosine and Stavudine
Experimental evidence demonstrated that methadone decreased the area under the concentration-time curve (AUC) and peak levels for didanosine and stavudine, with a more significant decrease for didanosine. Methadone disposition was not substantially altered.Zidovudine
Experimental evidence demonstrated that methadone increased the AUC of zidovudine which could result in toxic effects.Effects of Methadone on Antidepressants
Desipramine
Plasma levels of desipramine have increased with concurrent methadone administration.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The majority of available data from clinical trials, observational studies, case series, and case reports on methadone use in pregnancy do not indicate an increased risk of major malformations specifically due to methadone.Pregnant women involved in methadone maintenance programs have been reported to have improved prenatal care leading to reduced incidence of obstetric and fetal complications and neonatal morbidity and mortality when compared to women using illicit drugs. Several factors, including maternal use of illicit drugs, nutrition, infection and psychosocial circumstances, complicate the interpretation of investigations of the children of women who take methadone during pregnancy. Information is limited regarding dose and duration of methadone use during pregnancy, and most maternal exposure in these studies appears to occur after the first trimester of pregnancy (see Data).
Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy [see Warnings and Precautions (5.6)].
In published animal reproduction studies, methadone administered subcutaneously during the early gestational period produced neural tube defects (i.e., exencephaly and cranioschisis) in the hamster at doses 2 times the human daily oral dose of 120 mg/day on a mg/m2 basis (HDD) and in mice at doses equivalent to the HDD. Administration of methadone to pregnant animals during organogenesis and through lactation resulted decreased litter size, increased pup mortality, decreased pup body weights, developmental delays, and long-term neurochemical changes in the brain of offspring which correlate with altered behavioral responses that persist through adulthood at exposures comparable to and less than the HDD. Administration of methadone to male rodents prior to mating with untreated females resulted in increased neonatal mortality and significant differences in behavioral tests in the offspring at exposures comparable to and less than the HDD (see Data). Based on animal data, advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and Embryo-Fetal Risk
Untreated opioid addiction in pregnancy is associated with adverse obstetrical outcomes such as low birth weight, preterm birth, and fetal death. In addition, untreated opioid addiction often results in continued or relapsing illicit opioid use.Dosage Adjustment During Pregnancy
Dosage adjustment using higher doses or administering the daily dose in divided doses may be necessary in pregnant women treated with Methadose. Pregnant women appear to have significantly lower trough plasma methadone concentrations, increased plasma methadone clearance, and shorter methadone half-life than after delivery [see Dosage and Administration (2.9), Clinical Pharmacology (12.3)]. Withdrawal signs and symptoms should be closely monitored and the dose adjusted as necessary.Fetal/Neonatal Adverse Reactions
Neonatal opioid withdrawal syndrome may occur in newborn infants of mothers who are receiving treatment with METHADOSE.Neonatal opioid withdrawal syndrome presents as irritability, hyperactivity and abnormal sleep pattern, high-pitched cry, tremor, vomiting, diarrhea, and/or failure to gain weight. Signs of neonatal withdrawal usually occur in the first days after birth. The duration and severity of neonatal opioid withdrawal syndrome may vary. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly [see Warnings and Precautions (5.6)].
Labor or Delivery
Opioid-dependent women on methadone maintenance therapy may require additional analgesia during labor.Monitor neonates exposed to opioid analgesics during labor for signs of excess sedation and respiratory depression.
Data
Human Data
The majority of available data from clinical trials, observational studies, case series, and case reports on methadone use in pregnancy do not indicate an increased risk of major malformations specifically due to methadone. Findings regarding specific major malformations, decreased fetal growth, premature birth and Sudden Infant Death Syndrome have been inconsistent. Children prenatally exposed to methadone have been reported to demonstrate mild but persistent deficits in performance on psychometric and behavioral tests and visual abnormalities.In a multicenter, double-blind, randomized, controlled trial [Maternal Opioid Treatment: Human Experimental Research (MOTHER)] designed primarily to assess neonatal opioid withdrawal effects, opioid-dependent pregnant women were randomized to buprenorphine (n=86) or methadone (n=89) treatment, with enrollment at an average gestational age of 18.7 weeks in both groups. A total of 28 of the 86 women in the buprenorphine group (33%) and 16 of the 89 women in the methadone group (18%) discontinued treatment before the end of pregnancy.
Among women who remained in treatment until delivery, there was no difference between methadone-treated and buprenorphine-treated groups in the number of neonates requiring NOWS treatment or in the peak severity of NOWS. Buprenorphine-exposed neonates required less morphine (mean total dose, 1.1 mg vs. 10.4 mg), had shorter hospital stays (10.0 days vs. 17.5 days), and shorter duration of treatment for NOWS (4.1 days vs. 9.9 days) compared to the methadone-exposed group. There were no differences between groups in other primary outcomes (neonatal head circumference,) or secondary outcomes (weight and length at birth, preterm birth, gestational age at delivery, and
1-minute and 5-minute Apgar scores), or in the rates of maternal or neonatal adverse events. The outcomes among mothers who discontinued treatment before delivery and may have relapsed to illicit opioid use are not known. Because of the imbalance in discontinuation rates between the methadone and buprenorphine groups, the study findings are difficult to interpret.Animal Data
Formal reproductive and developmental toxicology studies for methadone have not been conducted. Exposure margins for the following published study reports are based on a human daily dose (HDD) of 120 mg methadone using a body surface area comparison.In a published study in pregnant hamsters, a single subcutaneous dose of methadone ranging from 31 mg/kg (2 times the HDD) to 185 mg/kg on Gestation Day 8 resulted in a decrease in the number of fetuses per litter and an increase in the percentage of fetuses exhibiting neural tube defects including exencephaly, cranioschisis, and “various other lesions.” The majority of the doses tested also resulted in maternal death. In a study in pregnant JBT/Jd mice, a single subcutaneous dose of 22 to 24 mg/kg methadone (approximately equivalent to the HDD) administered on Gestation Day 9 produced exencephaly in 11% of the embryos. In another study in pregnant mice, subcutaneous doses up to 28 mg/kg/day methadone (equivalent to the HDD) administered from Gestation Day 6 to 15 resulted in no malformations, but there were increased postimplantation loss and decreased live fetuses at 10 mg/kg/day or greater (0.4 times the HDD) and decreased ossification and fetal body weight at 20 mg/kg/day or greater (0.8 times the HDD). In a second study of pregnant mice dosed with subcutaneous doses up to 28 mg/kg/day methadone from Gestation Day 6 to 15, there was decreased pup viability, delayed onset of development of negative phototaxis and eye opening, increased righting reflexes at 5 mg/kg/day or greater (0.2 times the HDD), and decreased number of live pups at birth and decreased pup weight gain at 20 mg/kg/day or greater (0.8 times the HDD).
No effects were reported in a study of pregnant rats and rabbits at oral doses up to 40 mg/kg (3 and 6 times, respectively, the HDD) administered from Gestation Days 6 to 15 and 6 to 18, respectively.
When pregnant rats were treated with intraperitoneal doses of 2.5, 5, or 7.5 mg/kg methadone from one week prior to mating, through gestation until the end of lactation period, 5 mg/kg or greater (0.4 times the HDD) methadone resulted in decreases in litter size and live pups born and 7.5 mg/kg (0.6 times the HDD) resulted in decreased birth weights. Furthermore, decreased pup viability and pup body weight gain at 2.5 mg/kg or greater (0.2 times the HDD) were noted during the preweaning period.
Additional animal data demonstrate evidence for neurochemical changes in the brains of offspring from methadone-treated pregnant rats, including changes to the cholinergic, dopaminergic, noradrenergic, and serotonergic systems at doses below the HDD. Other animal studies have reported that prenatal and/or postnatal exposure to opioids including methadone alters neuronal development and behavior in the offspring including alterations in learning ability, motor activity, thermal regulation, nociceptive responses, and sensitivity to drugs at doses below the HDD. Treatment of pregnant rats subcutaneously with 5 mg/kg methadone from Gestation Day 14 to 19 (0.4 times the HDD) reduced fetal blood testosterone and androstenedione in males.
Published animal data have reported increased neonatal mortality in the offspring of male rodents that were treated with methadone at doses comparable to and less than the HDD for 1 to 12 days before and/or during mating (with more pronounced effects in the first 4 days). In these studies, the female rodents were not treated with methadone, indicating paternally-mediated developmental toxicity. Specifically, methadone administered to the male rat prior to mating with methadone-naïve females resulted in decreased weight gain in progeny after weaning. The male progeny demonstrated reduced thymus weights, whereas the female progeny demonstrated increased adrenal weights. Behavioral testing of these male and female progeny revealed significant differences in behavioral tests compared to control animals, suggesting that paternal methadone exposure can produce physiological and behavioral changes in progeny in this model. Examination of uterine contents of methadone-naïve female mice bred to methadone-treated male mice (once a day for three consecutive days) indicated that methadone treatment produced an increase in the rate of preimplantation deaths in all post-meiotic states at 1 mg/kg/day or greater (0.04 times the HDD). Chromosome analysis revealed a dose-dependent increase in the frequency of chromosomal abnormalities at 1 mg/kg/day or greater.
Studies demonstrated that methadone treatment of male rats for 21 to 32 days prior to mating with methadone-naïve females did not produce any adverse effects, suggesting that prolonged methadone treatment of the male rat resulted in tolerance to the developmental toxicities noted in the progeny. Mechanistic studies in this rat model suggest that the developmental effects of “paternal” methadone on the progeny appear to be due to decreased testosterone production. These animal data mirror the reported clinical findings of decreased testosterone levels in human males on methadone maintenance therapy for opioid addiction and in males receiving chronic intraspinal opioids.
8.2 Lactation
Risk Summary
Based on two small clinical studies, methadone was present in low levels in human milk, but the exposed infants in these studies did not show adverse reactions. Based on an average milk consumption of150 mL/kg/day, an infant would consume approximately 17.4 mcg/kg/day which is approximately 2% to 3% of the oral maternal dose. There have been rare case reports of sedation and respiratory depression in infants exposed to methadone through breast milk (see Data). Monitor infants exposed to Methadose through breast milk for excess sedation and respiratory depression. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for methadone and any potential adverse effects on the breastfed child from the drug or from the underlying maternal condition.
Data
In a study of ten breastfeeding women maintained on oral methadone doses of 10 to 80 mg/day, methadone concentrations from 50 to 570 mcg/L in milk were reported, which, in the majority of samples, were lower than maternal serum drug concentrations at steady state. Peak methadone levels in milk occur approximately 4 to 5 hours after an oral dose.In a study of twelve breastfeeding women maintained on oral methadone doses of 20 to 80 mg/day, methadone concentrations from 39 to 232 mcg/L in milk were reported. Based on an average milk consumption of 150 mL/kg/day, an infant would consume approximately 17.4 mcg/kg/day, which is approximately 2% to 3% of the oral maternal dose. Methadone has been detected in very low plasma concentrations in some infants whose mothers were taking methadone.
8.3 Females and Males of Reproductive Potential
Infertility
The effect of METHADOSE on fertility is unknown. Chronic use of opioids may cause reduced fertility in females and males of reproductive potential. It is not known whether these effects on fertility are reversible [see Adverse Reactions (6), Clinical Pharmacology (12.2), Nonclinical Toxicology (13)]. Reproductive function in human males may be decreased by methadone treatment. Reductions in ejaculate volume and seminal vesicle and prostate secretions have been reported in methadone-treated individuals. In addition, reductions in serum testosterone levels and sperm motility, and abnormalities in sperm morphology have been reported.In published animal studies, methadone produces a significant regression of sex accessory organs and testes of male mice and rats and administration of methadone to pregnant rats reduced fetal blood testosterone and androstenedione in male offspring [see Nonclinical Toxicology (13)].
8.4 Pediatric Use
The safety, effectiveness, and pharmacokinetics of methadone in pediatric patients below the age of 18 years have not been established.
8.5 Geriatric Use
Clinical studies of methadone did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently compared to younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, start elderly patients at the low end of the dosing range, taking into account the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy. Closely monitor elderly patients for signs of respiratory and central nervous system depression.
Methadone is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Hepatic Impairment
Methadone pharmacokinetics have not been extensively evaluated in patients with hepatic insufficiency. Methadone is metabolized by hepatic pathways, therefore, patients with liver impairment may be at risk of increased systemic exposure to methadone after multiple dosing. Start these patients on lower doses and titrate slowly while carefully monitoring for signs of respiratory and central nervous system depression.
8.7 Renal Impairment
Methadone pharmacokinetics have not been extensively evaluated in patients with renal insufficiency. Since unmetabolized methadone and its metabolites are excreted in urine to a variable degree, start these patients on lower doses and with longer dosing intervals and titrate slowly while carefully monitoring for signs of respiratory and central nervous system depression.
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
METHADOSE contains methadone, a substance with a high potential for abuse similar to other opioids including fentanyl, hydrocodone, hydromorphone, morphine, oxycodone, oxymorphone, and tapentadol. METHADOSE can be abused and is subject to misuse, addiction, and criminal diversion [see Warnings and Precautions (5.5)].
Prescription drug abuse is the intentional non-therapeutic use of a prescription drug, even once, for its rewarding psychological or physiological effects.
Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that develop after repeated substance use and includes: a strong desire to take the drug, difficulties in controlling its use, persisting in its use despite harmful consequences, a higher priority given to drug use than to other activities and obligations, increased tolerance, and sometimes a physical withdrawal.
“Drug-seeking” behavior is very common in persons with substance use disorders. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with prescriptions, and reluctance to provide prior medical records or contact information for other treating healthcare provider(s). “Doctor shopping” (visiting multiple prescribers to obtain additional prescriptions) is common among people who abuse opioids and people suffering from untreated addiction. Abuse and addiction are separate and distinct from physical dependence and tolerance. Healthcare providers should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all people with substance use disorders. In addition, abuse of opioids can occur in the absence of true addiction.
METHADOSE, like other opioids, can be diverted for non-medical use into illicit channels of distribution. Careful record-keeping of prescribing information, including quantity and frequency as required by state and federal law, is strongly advised.
Proper assessment and selection of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Risks Specific to METHADOSE
Abuse of methadone poses a risk of overdose and death. This risk is increased with concurrent abuse of methadone with alcohol and other substances. METHADOSE is intended for oral use only and must not be injected. Parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.METHADOSE, when used for the treatment of opioid addiction in detoxification or maintenance programs, may be dispensed only by opioid treatment programs certified by the Substance Abuse and Mental Health Services Administration (and agencies, practitioners, and institutions by formal agreements with the program sponsor).
9.3 Dependence
Both tolerance and physical dependence can develop during chronic opioid therapy. Tolerance is the need for increasing doses of opioids to maintain a defined effect (in the absence of disease progression or other external factors). Tolerance may occur to both the desired and undesired effects of drugs, and may develop at different rates for different effects.
Physical dependence results in withdrawal symptoms after abrupt discontinuation or significant dose reduction of a drug. Withdrawal is also precipitated through the administration of drugs with opioid antagonist activity (e.g., naloxone) or mixed agonist/antagonist analgesics (e.g., pentazocine, butorphanol, nalbuphine), or partial agonists (e.g., buprenorphine). Physical dependence may not occur to a clinically significant degree until after several days to weeks of continued opioid usage. Physical dependence is expected during opioid agonist therapy of opioid addiction.
METHADOSE should not be abruptly discontinued [see Dosage and Administration (2.6, 2.7)]. If METHADOSE is abruptly discontinued in a physically dependent patient, a withdrawal syndrome may occur. Some or all of the following can characterize this syndrome: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate [see Dosage and Administration (2.6)].
Neonatal opioid withdrawal syndrome (NOWS) is an expected and treatable outcome of prolonged use of opioids during pregnancy [see Warnings and Precautions (5.6)].
-
10 OVERDOSAGE
Clinical Presentation
Acute overdosage with methadone can be manifested by respiratory depression somnolence progressing to stupor or coma, skeletal-muscle flaccidity, cold and clammy skin, constricted pupils, and, in some cases, pulmonary edema, bradycardia, hypotension, hypoglycemia, partial or complete airway obstruction, atypical snoring, and death. Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations [see Clinical Pharmacology (12.2)]. In severe overdosage, particularly by the intravenous route, apnea, circulatory collapse, cardiac arrest, and death may occur.Methadone overdosage is associated with rhabdomyolysis. Seek medical attention, especially if abuse/misuse results in prolonged immobilization. Acute toxic leukoencephalopathy has been reported after methadone overdose, often weeks after apparent recovery from the initial intoxication. Hearing loss has been reported after methadone overdose, in some cases permanent.
Treatment of Overdose
In the case of overdose, priorities are the reestablishment of a patent and protected airway and institution of assisted or controlled ventilation, if needed. Employ other supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life-support techniques.Opioid antagonists, such as naloxone, are specific antidotes to respiratory depression resulting from opioid overdose. For clinically significant respiratory or circulatory depression secondary to opioid overdose, administer an opioid antagonist.
Because the duration of opioid reversal is expected to be less than the duration of action of methadone, carefully monitor the patient until spontaneous respiration is reliably established. If the response to an opioid antagonist is suboptimal or not sustained, administer additional antagonist as directed in the product’s prescribing information.
In an individual physically dependent on opioids, the administration of the usual dose of an opioid antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal symptoms experienced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be initiated with care and by titration with smaller than usual doses of the antagonist.
-
11 DESCRIPTION
METHADOSE™ (methadone hydrochloride, USP) oral concentrate contains methadone, an opioid agonist, and is available as a cherry-flavored liquid concentrate for oral administration. METHADOSE™ (methadone hydrochloride, USP) sugar-free oral concentrate is a dye-free, sugar-free, unflavored liquid concentrate of methadone hydrochloride for oral administration.
Methadone hydrochloride is chemically described as 6-(dimethylamino)-4,4-diphenyl-3-heptone hydrochloride. Methadone hydrochloride, USP is a fine white powder. It is very soluble in water, soluble in isopropanol and in chloroform, and practically insoluble in ether and in glycerine. It is present in METHADOSE as the racemic mixture. Methadone hydrochloride has a melting point of 235°C, a pKa of 8.25 in water at 20°C, a solution (1 part per 100) pH between 4.5 and 6.5, a partition coefficient of 117 at pH 7.4 in octanol/water and a molecular weight of 345.91. Its structural formula is C21H27NO•HCl.
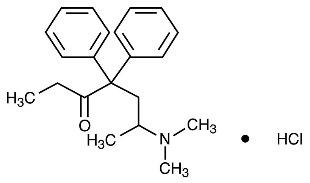
Each liquid concentrate contains 10 mg of methadone hydrochloride (equivalent to 8.95 mg of methadone) per mL and the following inactive ingredients: artificial cherry flavor, citric acid anhydrous, FD&C Red No 40, D&C Red No 33, methylparaben, poloxamer 407, propylene glycol, propylparaben, purified water, sodium citrate dihydrate, sucrose.
Other ingredients of METHADOSE sugar-free oral concentrate: citric acid anhydrous, purified water, sodium benzoate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Methadone hydrochloride is a mu-agonist; a synthetic opioid with multiple actions qualitatively similar to those of morphine, the most prominent of which involves the central nervous system and organs composed of smooth muscle. The methadone withdrawal syndrome, although qualitatively similar to that of morphine, differs in that the onset is slower, the course is more prolonged, and the symptoms are less severe.
Some data also indicate that methadone acts as an antagonist at the N-methyl-D-aspartate (NMDA) receptor. The contribution of NMDA receptor antagonism to methadone’s efficacy is unknown. Other NMDA receptor antagonists have been shown to produce neurotoxic effects in animals.
12.2 Pharmacodynamics
Effects on the Central Nervous System
Methadone produces respiratory depression by direct action on brain stem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to both increases in carbon dioxide tension and electrical stimulation.Methadone causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origins may produce similar findings). Marked mydriasis rather than miosis may be seen due to hypoxia in overdose situations.
Some NMDA receptor antagonists have been shown to produce neurotoxic effects in animals.
Effects on the Gastrointestinal Tract and Other Smooth Muscle
Methadone causes a reduction in motility associated with an increase in smooth muscle tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone is increased to the point of spasm, resulting in constipation. Other opioid-induced effects may include a reduction in biliary and pancreatic secretions, spasm of sphincter of Oddi, and transient elevations in serum amylase.Effects on the Cardiovascular System
Methadone produces peripheral vasodilation, which may result in orthostatic hypotension or syncope. Manifestations of histamine release and/or peripheral vasodilation may include pruritus, flushing, red eyes, sweating, and/or orthostatic hypotension.Effects on the Endocrine System
Opioids inhibit the secretion of adrenocorticotropic hormone (ACTH), cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon.Chronic use of opioids may influence the hypothalamic-pituitary-gonadal axis, leading to androgen deficiency that may manifest as low libido, impotence, erectile dysfunction, amenorrhea, or infertility. The causal role of opioids in the clinical syndrome of hypogonadism is unknown because the various medical, physical, lifestyle, and psychological stressors that may influence gonadal hormone levels have not been adequately controlled for in studies conducted to date [see Adverse Reactions (6)].
Effects on the Immune System
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown. Overall, the effects of opioids appear to be modestly immunosuppressive.Concentration-Adverse Reaction Relationships
There is a relationship between increasing methadone plasma concentration and increasing frequency of dose-related opioid adverse reactions such as nausea, vomiting, CNS effects, and respiratory depression. In opioid-tolerant patients, the situation may be altered by the development of tolerance to opioid-related adverse reactions [see Dosage and Administration (2.4, 2.5, 2.6)].12.3 Pharmacokinetics
Absorption
Following oral administration the bioavailability of methadone ranges between 36% to 100% and peak plasma concentrations are achieved between 1 and 7.5 hours. Dose proportionality of methadone pharmacokinetics is not known. However, after administration of daily oral doses ranging from 10 to 225 mg, the steady-state plasma concentrations ranged between 65 to 630 ng/mL and the peak concentrations ranged between 124 to 1255 ng/mL. Effect of food on the bioavailability of methadone has not been evaluated.Distribution
Methadone is a lipophilic drug and the steady-state volume of distribution ranges between 1.0 to 8.0 L/kg. In plasma, methadone is predominantly bound to α1-acid glycoprotein (85% to 90%). Methadone is secreted in saliva, breast milk, amniotic fluid and umbilical cord plasma.Elimination
Metabolism
Methadone is primarily metabolized by N-demethylation to an inactive metabolite, 2-ethylidene-1,5-dimethyl-3,3-diphenylpyrrolidene (EDDP). Cytochrome P450 enzymes, primarily CYP3A4, CYP2B6, CYP2C19, CYP2C9 and CYP2D6, are responsible for conversion of methadone to EDDP and other inactive metabolites, which are excreted mainly in the urine.Excretion
The elimination of methadone is mediated by extensive biotransformation, followed by renal and fecal excretion. Published reports indicate that after multiple dose administration the apparent plasma clearance of methadone ranged between 1.4 and 126 L/h, and the terminal half-life (T1/2) was highly variable and ranged between 8 and 59 hours in different studies. Methadone is a basic (pKa=9.2) compound and the pH of the urinary tract can alter its disposition in plasma. Urine acidification has been shown to increase renal elimination of methadone. Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for increasing the elimination of methadone or its metabolites. Also, since methadone is lipophilic, it has been known to persist in the liver and other tissues. The slow release from the liver and other tissues may prolong the duration of methadone action despite low plasma concentrations.Drug Interaction Studies
Cytochrome P450 Interactions
Methadone undergoes hepatic N-demethylation by cytochrome P450 isoforms, principally CYP3A4, CYP2B6, CYP2C19, CYP2C9 and CYP2D6. Coadministration of methadone with CYP inducers may result in more rapid metabolism and decreased effects of methadone, whereas administration with CYP inhibitors may reduce metabolism and potentiate methadone’s effects. Pharmacokinetics of methadone may be unpredictable when coadministered with drugs that are known to both induce and inhibit CYP enzymes. Although anti-retroviral drugs such as efavirenz, nelfinavir, nevirapine, ritonavir, and lopinavir+ritonavir combination are known to inhibit some CYPs, they are shown to reduce the plasma levels of methadone, possibly due to their CYP induction activity [see Drug Interactions (7)].Cytochrome P450 Inducers
The following drug interactions were reported following coadministration of methadone with known inducers of cytochrome P450 enzymes:- Rifampin: In patients well-stabilized on methadone, concomitant administration of rifampin resulted in a marked reduction in serum methadone levels and a concurrent appearance of withdrawal symptoms.
- Phenytoin: In a pharmacokinetic study with patients on methadone maintenance therapy, phenytoin administration (250 mg twice daily initially for 1 day followed by 300 mg daily for 3 to 4 days) resulted in an approximately 50% reduction in methadone exposure and withdrawal symptoms occurred concurrently. Upon discontinuation of phenytoin, the incidence of withdrawal symptoms decreased and methadone exposure increased to a level comparable to that prior to phenytoin administration.
- St. John’s Wort, Phenobarbital, Carbamazepine: Administration of methadone with other CYP3A4 inducers may result in withdrawal symptoms.
Cytochrome P450 Inhibitors
- Voriconazole: Voriconazole can inhibit the activity of CYP3A4, CYP2C9 and CYP2C19. Repeat dose administration of oral voriconazole (400 mg every 12 hours for 1 day, then 200 mg every 12 hours for 4 days) increased the Cmax and AUC of (R)-methadone by 31% and 47%, respectively, in subjects receiving a methadone maintenance dose (30 to 100 mg daily). The Cmax and AUC of (S)-methadone increased by 65% and 103%, respectively. Increased plasma concentrations of methadone have been associated with toxicity, including QT prolongation. Frequent monitoring for adverse events and toxicity related to methadone is recommended during coadministration. Dose reduction of methadone may be needed [see Drug Interactions (7)].
Anti-Retroviral Agents
Although anti-retroviral drugs such as efavirenz, nelfinavir, nevirapine, ritonavir, and lopinavir+ritonavir combination are known to inhibit CYPs, they are shown to reduce the plasma levels of methadone, possibly due to their CYP induction activity.- Abacavir, Amprenavir, Efavirenz, Nelfinavir, Nevirapine, Ritonavir, Lopinavir+Ritonavir Combination: Coadministration of these anti-retroviral agents resulted in increased clearance or decreased plasma levels of methadone.
- Didanosine and Stavudine: Experimental evidence demonstrated that methadone decreased the area under the concentration-time curve (AUC) and peak levels for didanosine and stavudine, with a more significant decrease for didanosine. Methadone disposition was not substantially altered.
- Zidovudine: Experimental evidence demonstrated that methadone increased the AUC of zidovudine which could result in toxic effects.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The results of carcinogenicity assessment in B6C2F1 mice and Fischer 344 rats following dietary administration of two doses of methadone HCl have been published. Mice consumed 15 mg/kg/day or 60 mg/kg/day methadone for two years. These doses were approximately 0.6 and 2.5 times a human daily oral dose of 120 mg/day on a body surface area basis (HDD). There was a significant increase in pituitary adenomas in female mice treated with 15 mg/kg/day but not with 60 mg/kg/day. Under the conditions of the assay, there was no clear evidence for a treatment-related increase in the incidence of neoplasms in male rats. Due to decreased food consumption in males at the high dose, male rats consumed 16 mg/kg/day and 28 mg/kg/day of methadone for two years. These doses were approximately 1.3 and 2.3 times the HDD. In contrast, female rats consumed 46 mg/kg/day or 88 mg/kg/day for two years. These doses were approximately 3.7 and 7.1 times the HDD. Under the conditions of the assay, there was no clear evidence for a treatment-related increase in the incidence of neoplasms in either male or female rats.Mutagenesis
There are several published reports on the potential genetic toxicity of methadone. Methadone tested positive in the in vivo mouse dominant lethal assay and the in vivo mammalian spermatogonial chromosome aberration test. Additionally, methadone tested positive in the E. coli DNA repair system and Neurospora crassa and mouse lymphoma forward mutation assays. In contrast, methadone tested negative in tests for chromosome breakage and disjunction and sex-linked recessive lethal gene mutations in germ cells of Drosophila using feeding and injection procedures.Impairment of Fertility
Published animal studies provide additional data indicating that methadone treatment of males can alter reproductive function. Methadone produces decreased sexual activity (mating) of male rats at 10 mg/kg/day (corresponding to 0.3 times the human daily oral dose of 120 mg/day based on body surface area). Methadone also produces a significant regression of sex accessory organs and testes of male mice and rats at 0.2 and 0.8 times the HDD, respectively. Methadone treatment of pregnant rats from Gestation Day 14 to 19 reduced fetal blood testosterone and androstenedione in males. Decreased serum levels of testosterone were observed in male rats that were treated with methadone (1.3 to 3.3 mg/kg/day for 14 days, corresponding to 0.1 to 0.3 times the HDD) or 10 to 15 mg/kg/day for 10 days (0.8 to 1.2 times the HDD). -
16 HOW SUPPLIED/STORAGE AND HANDLING
METHADOSE™ (methadone hydrochloride, USP) oral concentrate 10 mg per mL is supplied as a red, cherry-flavored liquid concentrate.
1-Liter Bottle................ NDC 0406-0527-10
METHADOSE™ (methadone hydrochloride, USP) sugar-free oral concentrate 10 mg per mL is supplied as a dye-free, sugar-free, unflavored clear liquid concentrate.
1-Liter Bottle................ NDC 0406-8725-10
Dispense in tight containers, protected from light. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Store METHADOSE securely and dispose of properly [see Patient Counseling Information (17)].
-
17 PATIENT COUNSELING INFORMATION
Life-Threatening Respiratory Depression
Discuss the risk of respiratory depression with patients, explaining that the risk is greatest when starting METHADOSE or when the dose is increased.Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help right away in the event of a known or suspected overdose [see Warnings and Precautions (5.1)].
Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose
Because patients being treated with methadone may be at risk for opioid overdose during initiation or titration, or in the case of relapse to illicit use, discuss the importance of having access to naloxone with the patient and caregiver. Also discuss the importance of having access to naloxone if the patient has household members (including children) or other close contacts at risk for accidental ingestion or opioid overdose.Inform patients and caregivers of the options for obtaining naloxone as permitted by individual state naloxone dispensing and prescribing requirements and guidelines (e.g., by prescription, directly from a pharmacist, or as part of a community-based program).
Educate patients and caregivers on how to recognize the signs and symptoms of an opioid overdose.
Explain to patients and caregivers that naloxone’s effects are temporary, and that they must call 911 or get emergency medical help right away in all cases of known or suspected opioid overdose, even if naloxone is administered. Repeat administration may be necessary, particularly for overdose involving METHADOSE, because naloxone is often not effective at the doses available for patient access [see Dosage and Administration (2.3), Warnings and Precautions (5.1), Overdosage (10)].
If naloxone is prescribed, also advise patients and caregivers:
- How to treat with naloxone in the event of an opioid overdose
- To tell family and friends about their naloxone and to keep it in a place where family and friends can access it in an emergency
- To read the Patient Information (or other educational material) that will come with their naloxone. Emphasize the importance of doing this before an opioid emergency happens, so the patient and caregiver will know what to do.
Interactions with Benzodiazepines and Other CNS Depressants
Inform patients and caregivers that potentially fatal additive effects may occur if METHADOSE is used with benzodiazepines or other CNS depressants, including alcohol. Counsel patients that such medications should not be used concomitantly unless supervised by a healthcare provider [see Warnings and Precautions (5.2), Drug Interactions (7)].Symptoms of Arrhythmia
Instruct patients to seek medical attention immediately if they experience symptoms suggestive of an arrhythmia (such as palpitations, near syncope, or syncope) when taking METHADOSE [see Warnings and Precautions (5.3)].Accidental Ingestion
Inform patients that accidental ingestion, especially by children, may result in respiratory depression or death [see Warnings and Precautions (5.4)]. Instruct patients to take steps to store METHADOSE securely. Advise patients to dispose of unused METHADOSE by flushing down the toilet.Abuse Potential
Inform patients that METHADOSE contains methadone, a Schedule II controlled substance that is subject to abuse [see Warnings and Precautions (5.5)]. Instruct patients not to share METHADOSE with others and to take steps to protect METHADOSE from theft or misuse.Important Administration Instructions [see Dosage and Administration (2)]
Instruct patients how to properly take METHADOSE, including the following:- METHADOSE is for oral administration only. The preparation must not be injected.
- Inform patients that METHADOSE should be taken only as directed to reduce the risk of life-threatening adverse reactions (e.g., respiratory depression), and the dose should not be adjusted without consulting a physician or other healthcare professional.
- Reassure patients initiating treatment with METHADOSE for opioid dependence that the dose of methadone will “hold” for longer periods of time as treatment progresses.
- Apprise patients seeking to discontinue treatment with methadone for opioid dependence of the high risk of relapse to illicit drug use associated with discontinuation of METHADOSE maintenance treatment.
- Advise patients not to discontinue METHADOSE without first discussing the need for a tapering regimen with the prescriber.
Serotonin Syndrome
Inform patients that METHADOSE could cause a rare but potentially life-threatening condition resulting from concomitant administration of serotonergic drugs. Warn patients of the symptoms of serotonin syndrome and to seek medical attention right away if symptoms develop. Instruct patients to inform their physicians if they are taking, or plan to take, serotonergic medications [see Warnings and Precautions (5.9), Drug Interactions (7)].MAOI Interaction
Inform patients to avoid taking METHADOSE while using any drugs that inhibit monoamine oxidase. Patients should not start MAOIs while taking METHADOSE [see Drug Interactions (7)].Adrenal Insufficiency
Inform patients that METHADOSE could cause adrenal insufficiency, a potentially life-threatening condition. Adrenal insufficiency may present with non-specific symptoms and signs such as nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. Advise patients to seek medical attention if they experience a constellation of these symptoms [see Warnings and Precautions (5.10)].Anaphylaxis
Inform patients that anaphylaxis has been reported with ingredients contained in METHADOSE. Advise patients how to recognize such a reaction and when to seek medical attention [see Adverse Reactions (6)].Neonatal Opioid Withdrawal
Advise women that if they are pregnant while being treated with METHADOSE, the baby may have signs of withdrawal at birth and that withdrawal is treatable [see Warnings and Precautions (5.6), Use in Specific Populations (8.1)].Lactation
Advise women who are breastfeeding to monitor the infant for increased sleepiness (more than usual), difficulty breathing or limpness. Instruct nursing mothers using METHADOSE to watch for signs of methadone toxicity in their infants, which include increased sleepiness (more than usual), difficulty breastfeeding, breathing difficulties, or limpness. Instruct nursing mothers to talk to their baby’s healthcare provider immediately if they notice these signs. If they cannot reach the healthcare provider right away, instruct them to take the baby to the emergency room or call 911 (or local emergency services) [see Use in Specific Populations (8.2)].Infertility
Advise patients that chronic use of opioids, such as METHADOSE, may cause reduced fertility. It is not known whether these effects on fertility are reversible [see Use in Specific Populations (8.3)].Constipation
Advise patients of the potential for severe constipation, including management instructions and when to seek medical attention [see Adverse Reactions (6), Clinical Pharmacology (12.2)].Hypoglycemia
Inform patients that methadone may cause hypoglycemia. Instruct patients how to recognize the symptoms of low blood glucose and to contact their health care provider if these symptoms occur [see Warnings and Precautions (5.17)].Mallinckrodt, the “M” brand mark, the Mallinckrodt Pharmaceuticals logo and other brands are trademarks of a Mallinckrodt company.
© 2023 Mallinckrodt.
SpecGx LLC
Webster Groves, MO 63119 USA
Made in USAMallinckrodt™
Pharmaceuticals - How to treat with naloxone in the event of an opioid overdose
-
PRINCIPAL DISPLAY PANEL - Methadose™ Oral Concentrate 1 Liter Bottle Label
NDC 0406-0527-10
1 Liter (1000 mL)
Methadose™ Oral Concentrate
(methadone hydrochloride oral concentrate USP)CII
10 mg per mL Each milliliter contains:
Methadone Hydrochloride ............................. 10 mg
Methadose™ also contains Artificial Cherry Flavor, Citric Acid, FD&C Red 40, D&C Red 33, Methylparaben, Poloxamer 407, Propylene Glycol, Propylparaben, Purified Water, Sodium Citrate and Sucrose.
Rx only
DO NOT USE IF SEAL AROUND BOTTLE CAP IS BROKEN OR MISSING.
USUAL DOSE: To be determined by the physician; to be diluted with water or other liquid to 30 mL (1 fl. oz.) or more before oral administration.
Preserve in tight containers, protected from light. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Mallinckrodt™
GTIN: 00304060527102
L00M07 Rev 10/2018
SpecGx LLC, Webster Groves, MO 63119 USA
Made in USA

-
PRINCIPAL DISPLAY PANEL - Methadose™
Sugar-Free Oral Concentrate 1 Liter Bottle Label
NDC 0406-8725-10
1 Liter (1000 mL)
Methadose™ Sugar-Free Oral Concentrate
(methadone hydrochloride oral concentrate USP)
dye-free, sugar-free, unflavoredCII
10 mg per mL Each milliliter contains:
Methadone Hydrochloride ............................. 10 mgMethadose™ Sugar-Free Oral Concentrate also contains Citric Acid Anhydrous, Purified Water and Sodium Benzoate.
Rx only
DO NOT USE IF SEAL AROUND BOTTLE CAP IS BROKEN OR MISSING.
USUAL DOSE: To be determined by the physician; to be diluted with water or other liquid to 30 mL (1 fl. oz.) or more before oral administration.
Preserve in tight containers, protected from light. Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Mallinckrodt™
GTIN: 00304068725104
L00M22 Rev 10/2018
SpecGx LLC, Webster Groves, MO 63119 USA
Made in USA

-
INGREDIENTS AND APPEARANCE
METHADOSE
methadone hydrochloride concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0406-0527 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHADONE HYDROCHLORIDE (UNII: 229809935B) (METHADONE - UNII:UC6VBE7V1Z) METHADONE HYDROCHLORIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) D&C RED NO. 33 (UNII: 9DBA0SBB0L) METHYLPARABEN (UNII: A2I8C7HI9T) POLOXAMER 407 (UNII: TUF2IVW3M2) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCROSE (UNII: C151H8M554) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0406-0527-10 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/14/1973 2 NDC:0406-0527-15 15000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/14/1973 07/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017116 03/14/1973 METHADOSE SUGAR-FREE
methadone hydrochloride concentrateProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0406-8725 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHADONE HYDROCHLORIDE (UNII: 229809935B) (METHADONE - UNII:UC6VBE7V1Z) METHADONE HYDROCHLORIDE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0406-8725-10 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/14/1973 2 NDC:0406-8725-15 15000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/14/1973 07/31/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA017116 03/14/1973 Labeler - SpecGx LLC (080679498) Establishment Name Address ID/FEI Business Operations SpecGx LLC 957414238 analysis(0406-0527, 0406-8725) , manufacture(0406-0527, 0406-8725) Establishment Name Address ID/FEI Business Operations SpecGx LLC 163205300 api manufacture(0406-0527, 0406-8725)


