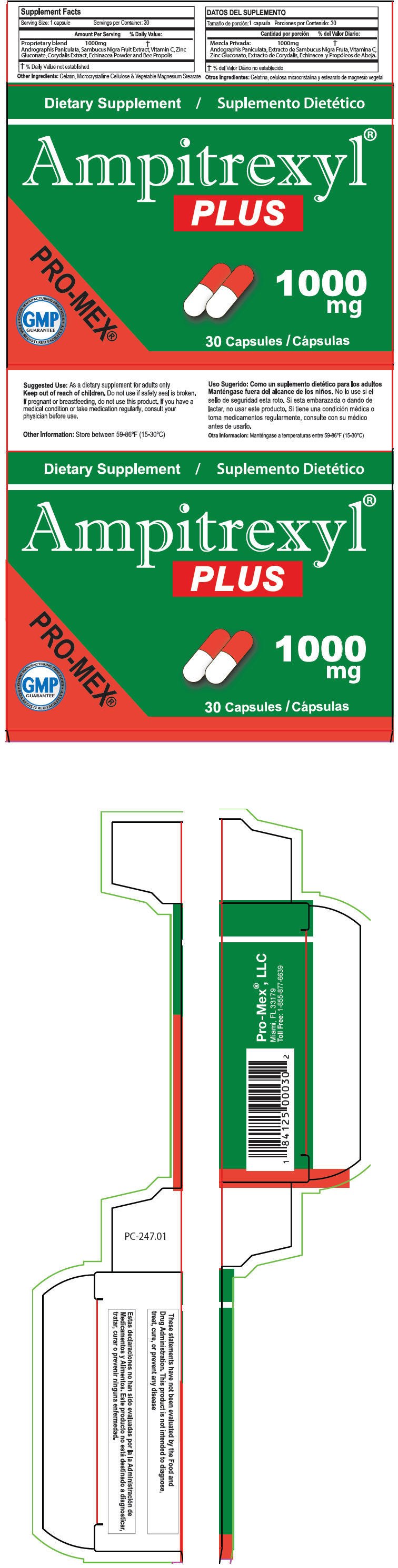
Label: AMPITREXYL PLUS- andrographis paniculata whole, european elderberry, ascorbic acid, zinc gluconate, dicentra canadensis root, echinacea, unspecified, and propolis wax capsule
- NHRIC Code(s): 58988-1830-1
- Packager: ProMex LLC
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated November 23, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
STATEMENT OF IDENTITY
Supplement Facts Serving Size: 1 capsule Servings per Container: 30 Amount Per Serving % Daily Value: - *
- % Daily Value not established
Proprietary blend 1000mg * Andrographis Paniculata, Sambucus Nigra Fruit Extract, Vitamin C, Zinc Gluconate, Corydalis Extract, Echinacea Powder and Bee Propolis Other Ingredients: Gelatin, Microcrystalline Cellulose & Vegetable Magnesium Stearate
- Suggested Use
- WARNINGS
- Other Information
- PRINCIPAL DISPLAY PANEL - 30 Capsule Blister Pack Carton
-
INGREDIENTS AND APPEARANCE
AMPITREXYL PLUS
andrographis paniculata whole, european elderberry, ascorbic acid, zinc gluconate, dicentra canadensis root, echinacea, unspecified, and propolis wax capsuleProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:58988-1830 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANDROGRAPHIS PANICULATA WHOLE (UNII: 0P49L952WZ) (ANDROGRAPHIS PANICULATA WHOLE - UNII:0P49L952WZ) ANDROGRAPHIS PANICULATA WHOLE 30 mg EUROPEAN ELDERBERRY (UNII: BQY1UBX046) (EUROPEAN ELDERBERRY - UNII:BQY1UBX046) EUROPEAN ELDERBERRY 250 mg ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 250 mg ZINC GLUCONATE (UNII: U6WSN5SQ1Z) (ZINC CATION - UNII:13S1S8SF37) ZINC GLUCONATE 75 mg DICENTRA CANADENSIS ROOT (UNII: Y5JS7YM46F) (DICENTRA CANADENSIS ROOT - UNII:Y5JS7YM46F) DICENTRA CANADENSIS ROOT 50 mg ECHINACEA, UNSPECIFIED (UNII: 4N9P6CC1DX) (ECHINACEA, UNSPECIFIED - UNII:4N9P6CC1DX) ECHINACEA, UNSPECIFIED 45 mg PROPOLIS WAX (UNII: 6Y8XYV2NOF) (PROPOLIS WAX - UNII:6Y8XYV2NOF) PROPOLIS WAX 30 mg Inactive Ingredients Ingredient Name Strength GELATIN (UNII: 2G86QN327L) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MAGNESIUM STEARATE (UNII: 70097M6I30) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:58988-1830-1 2 in 1 CARTON 1 15 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 05/31/2006 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color color scoring 1 shape size (solid drugs) 20 mm Labeler - ProMex LLC (789974388)