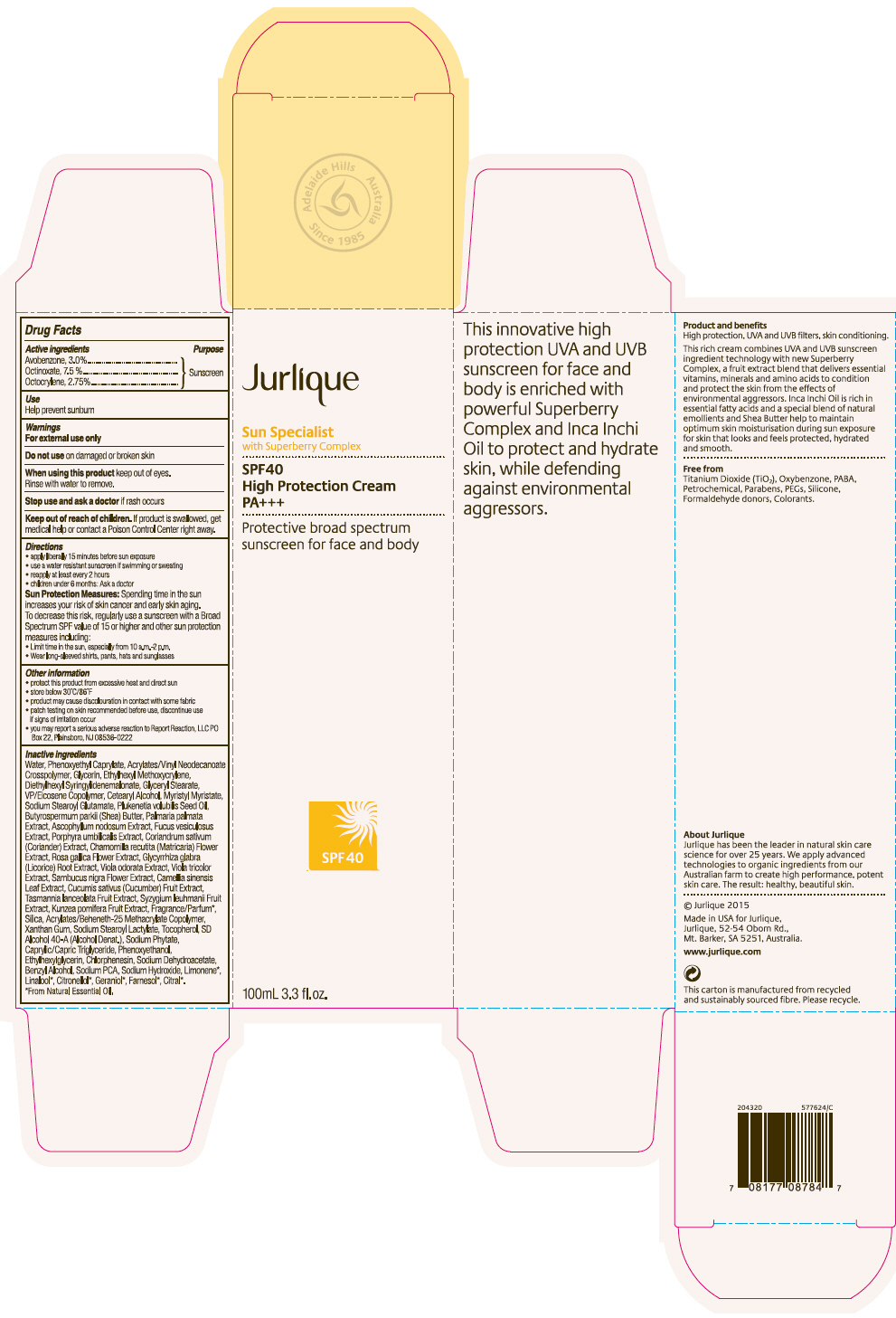
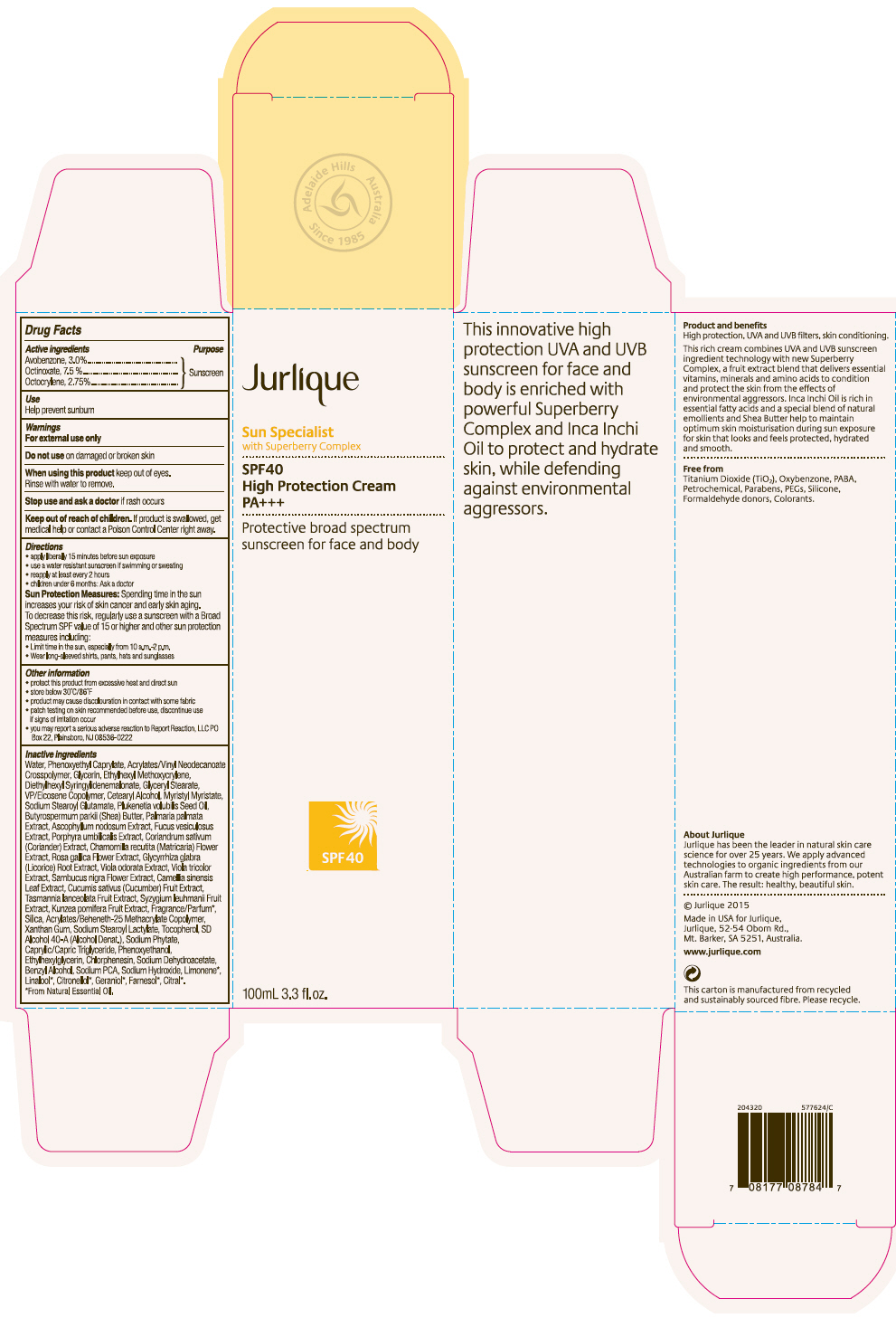
Label: JURLIQUE SPF40 HIGH PROTECTION SUNSCREEN- avobenzone, octinoxate, and octocrylene cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 68105-006-01 - Packager: Jurlique International
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 25, 2016
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- –
- Limit time in the sun, especially from 10 a.m.-2 p.m.
- –
- Wear long-sleeved shirts, pants, hats and sunglasses
-
Other information
- protect this product from excessive heat and direct sun
- Store below 30°C/86°F
- product may cause discolouration in contact with some fabric
- patch testing on skin recommended before use, discontinue use if signs of irritation occur
- you may report a serious adverse reaction to Report Reaction, LLC PO Box 22, Plainsboro, NJ 08536-022
-
Inactive ingredients
Water, Phenoxyethyl Caprylate, Acrylates/Vinyl Neodecanoate Crosspolymer, Glycerin, Ethylhexyl Methoxycrylene, Diethylhexyl Syringylidenemalonate, Glyceryl Stearate, VP/Eicosene Copolymer, Cetearyl Alcohol, Myristyl Myristate, Sodium Stearoyl Glutamate, Plukenetia volubilis Seed Oil, Butyrospermum parkii (Shea) Butter, Palmaria palmata Extract, Ascophyllum nodosum Extract, Fucus vesiculosus Extract, Porphyra umbilicalis Extract, Corandrum sativum (Coriander) Extract, Chamomilla recutita (Matricaria) Flower Extract, Rosa gallica Flower Extract, Glycyrrhiza glabra (Licorice) Extract, Viola odorata Extract, Viola tricolor Extract, Sambucus nigra Flower Extract, Camellia sinensis Leaf Extract, Cucumis sativus (Cucumber) Fruit Extract, Tasmannia lanceolata Fruit Extract, Syzygium leuhmanii Extract, Kunzea pomifera Fruit Extract, Fragrance/Parfum1, Silica, Acrylates/Beheneth-25 methacrylate Copolymer, Xanthan Gum, Sodium Stearoyl Lactylate, Tocopherol, SD Alcohol 40-A (Alcohol Denat.), Sodium Phytate, Caprylic/Capric Triglyceride, Phenoxyethanol, Ethylhexylglycerin, Chlorphenesin, Sodium Dehydroacetate, Benzyl Alcohol, Sodium PCA, Sodium Hydroxide, Limonene1, Linalool1, Citronellol1, Geraniol1, Farnesol1, Citral1.
- 1
- From Essential Oil
- PRINCIPAL DISPLAY PANEL - 100 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
JURLIQUE SPF40 HIGH PROTECTION SUNSCREEN
avobenzone, octinoxate, and octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68105-006 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 27.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Phenoxyethyl Caprylate (UNII: GMI5AN7T8U) ETHYL ACRYLATE/METHACRYLIC ACID/STEARETH-20 METHACRYLATE COPOLYMER (UNII: EPA1872R1N) Glycerin (UNII: PDC6A3C0OX) Ethylhexyl Methoxycrylene (UNII: S3KFG6Q5X8) Glyceryl Monostearate (UNII: 230OU9XXE4) Copovidone K25-31 (UNII: D9C330MD8B) Cetostearyl Alcohol (UNII: 2DMT128M1S) Myristyl Myristate (UNII: 4042ZC00DY) Sodium Stearoyl Glutamate (UNII: 65A9F4P024) Ascophyllum nodosum (UNII: 168S4EO8YJ) Fucus vesiculosus (UNII: 535G2ABX9M) Porphyra Umbilicalis (UNII: 14AN0J70WO) Coriander (UNII: 1OV56052IK) Chamomile (UNII: FGL3685T2X) Rosa Gallica Flower (UNII: X8W61WUV70) Glycyrrhiza Glabra (UNII: 2788Z9758H) Viola Odorata (UNII: AET12U8B74) Viola tricolor (UNII: 9Q24RAI43V) European Elderberry (UNII: BQY1UBX046) Bancha Tea Leaf/Twig (UNII: EWI42IEH1C) Cucumber (UNII: YY7C30VXJT) Silicon Dioxide (UNII: ETJ7Z6XBU4) Xanthan Gum (UNII: TTV12P4NEE) Sodium Stearoyl Lactylate (UNII: IN99IT31LN) Tocopherol (UNII: R0ZB2556P8) Alcohol (UNII: 3K9958V90M) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) Medium-Chain Triglycerides (UNII: C9H2L21V7U) Phenoxyethanol (UNII: HIE492ZZ3T) Ethylhexylglycerin (UNII: 147D247K3P) Sodium Dehydroacetate (UNII: 8W46YN971G) Benzyl Alcohol (UNII: LKG8494WBH) Phytate Sodium (UNII: 88496G1ERL) Sodium Hydroxide (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68105-006-01 1 in 1 CARTON 01/01/2015 1 100 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 01/01/2015 Labeler - Jurlique International (752025791) Registrant - Cosmetic Laboratories of America (013696501) Establishment Name Address ID/FEI Business Operations Cosmetic Laboratories of America 013696501 MANUFACTURE(68105-006)