Label: EXTRA STRENGTH ANTACID ORIGINAL FLAVOR- aluminum hydroxide and magnesium carbonate tablet, chewable
- NDC Code(s): 63868-996-01
- Packager: CHAIN DRUG MARKETING ASSOCIATION INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 8, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
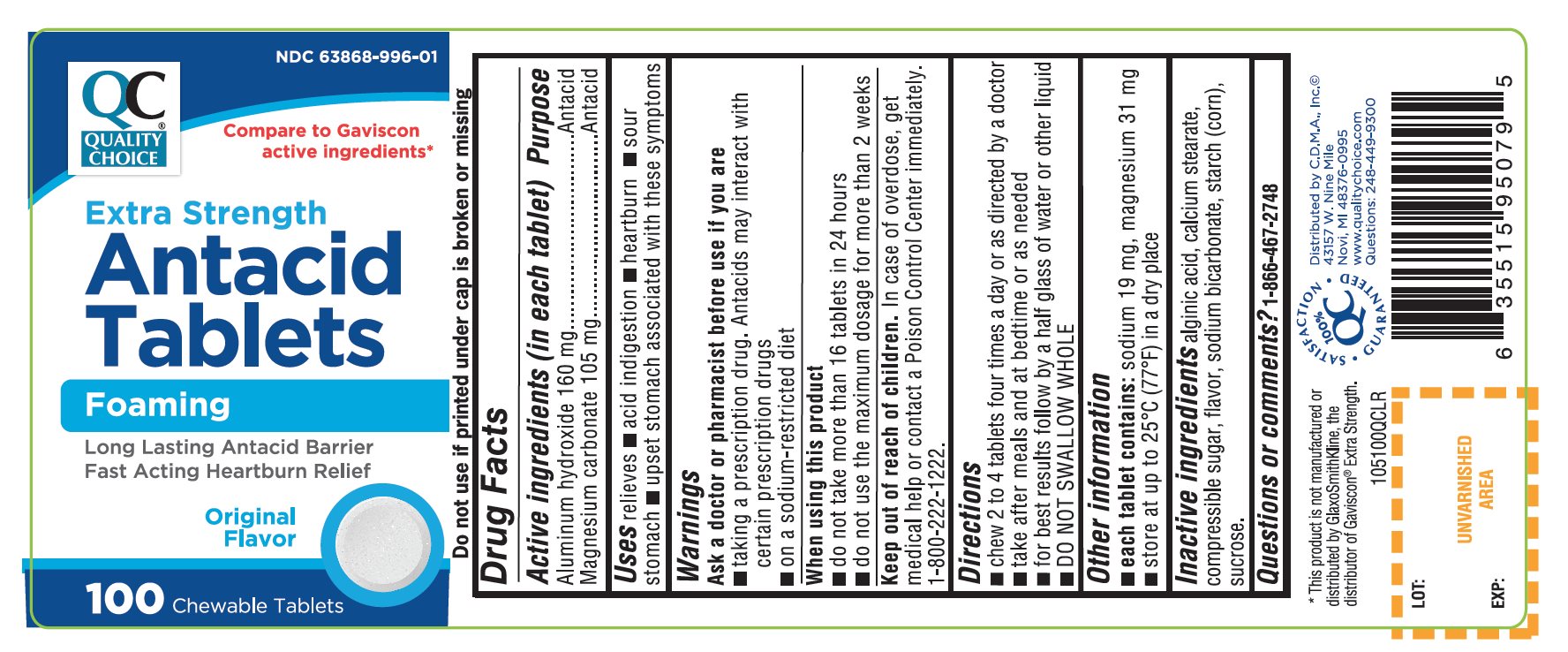
- Active ingredients (in each tablet)
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
-
Principal Display Panel
QUALITY CHOICE®
NDC 63868-996-01
Compare To Gaviscon® active ingredients*
Extra Strength
Antacid Tablets
Foaming
Long Lasting Antacid Barrier
Fast Acting Heartburn Relief
Original Flavor
100% QC SATISFACTION GUARANTEED
- DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS BROKEN OR MISSING.
- Distributed by C.D.M.A., Inc.©
- 43157 W. Nine Mile
- Novi, MI 48376-0995
- www.qualitychoice.com
- Questions: 248-449-9300
*This product is not manufactured or distributed by GlaxoSmithKline, the distributor of Gaviscon® Extra Strength.
-
INGREDIENTS AND APPEARANCE
EXTRA STRENGTH ANTACID ORIGINAL FLAVOR
aluminum hydroxide and magnesium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63868-996 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 160 mg MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 105 mg Inactive Ingredients Ingredient Name Strength ALGINIC ACID (UNII: 8C3Z4148WZ) CALCIUM STEARATE (UNII: 776XM7047L) CORN SYRUP (UNII: 9G5L16BK6N) SODIUM BICARBONATE (UNII: 8MDF5V39QO) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) Product Characteristics Color WHITE Score no score Shape ROUND Size 17mm Flavor BUTTERSCOTCH (ORIGINAL) Imprint Code RP105 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63868-996-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 04/19/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M001 04/19/2019 Labeler - CHAIN DRUG MARKETING ASSOCIATION INC. (011920774)